Antioxidant Activity and Phytochemical Composition of the Leaves of Solanum guaraniticum A. St.-Hil
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Composition
| Extract or Fraction | TP (mg GAE/g) | TF (mg RE/g) | CT (mg CaE/g) | TA (mg/g) |
|---|---|---|---|---|
| CE | 259.95 b ± 0.69 | 61.30 b ± 0.53 | 23.16 b ± 2.05 | 6.14 b ± 0.01 |
| CHCl3 | 195.90 c ± 1.24 | 75.73 a ± 0.34 | 56.03 a ± 0.68 | 10.79 a ± 0.06 |
| AcOEt | 546.57 a ± 2.35 | 57.17 c ± 0.07 | 11.85 c ± 0.91 | - |
| n-BuOH | 259.82 b ± 2.17 | 60.17 b ± 0.32 | 8.85 c ± 1.01 | - |
2.2. Radical Scavenging Capacity-DPPH Assay
2.3. Scavenging of ROS-DCFH-DA Method
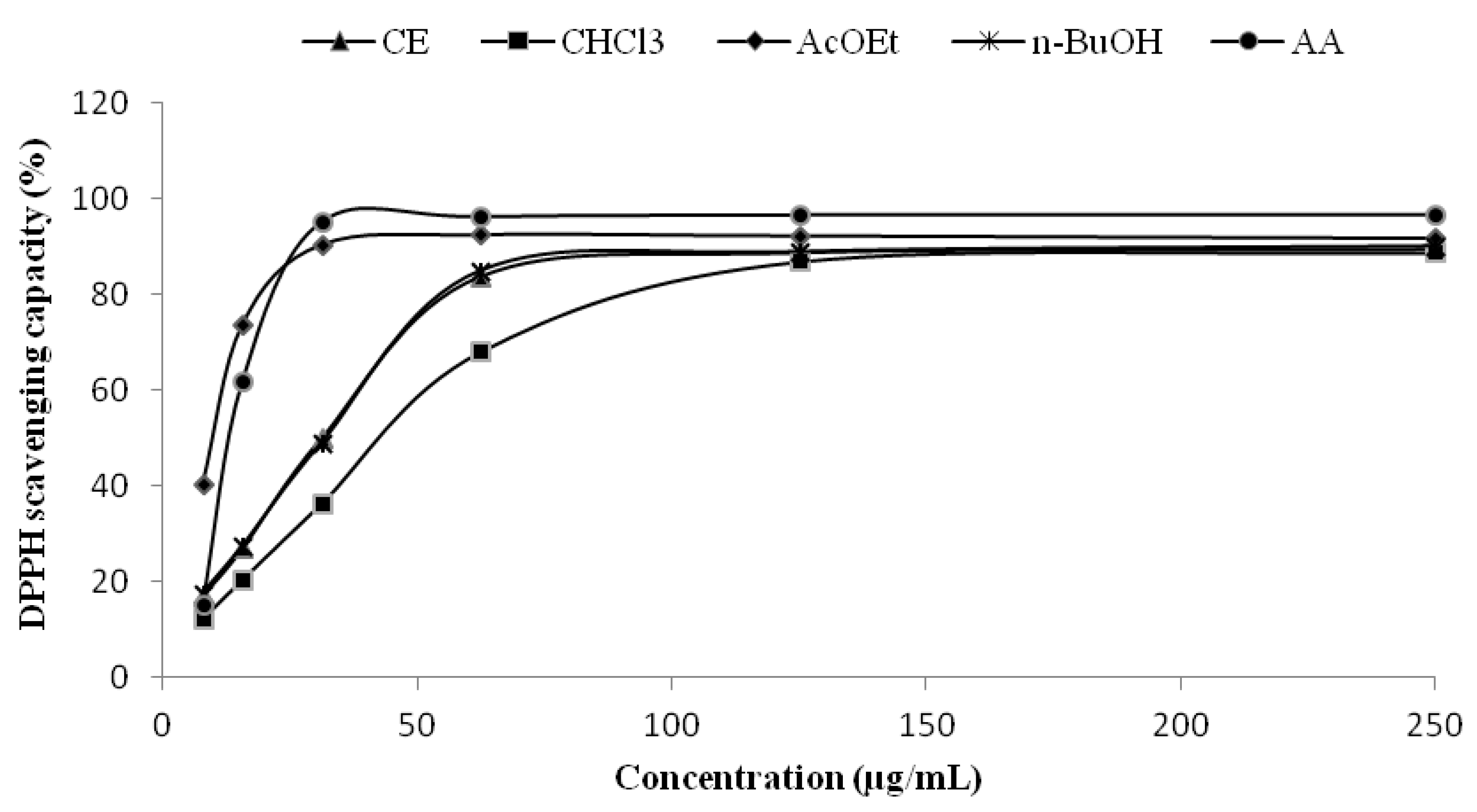
| Extract or Fraction | IC501 (µg/mL) | IC502 (µg/mL) | IC503 (µg/mL) |
|---|---|---|---|
| CE | 31.43 c ± 1.02 | 54.23 c ± 1.58 | 82.98 b ± 0.31 |
| CHCl3 | 44.46 d ± 1.27 | 3.85 a ± 0.93 | 67.65 a ± 0.82 |
| AcOEt | 9.11 a ± 0.75 | 12.24 b± 1.76 | 179.59 c ± 1.14 |
| n-BuOH | 32.12 c ± 0.91 | 55.10 c ± 1.94 | 87.22 b ± 0.32 |
| AA | 15.48 b ±1.28 | 117.81 d ± 1.23 | 61.80 a ± 0.54 |
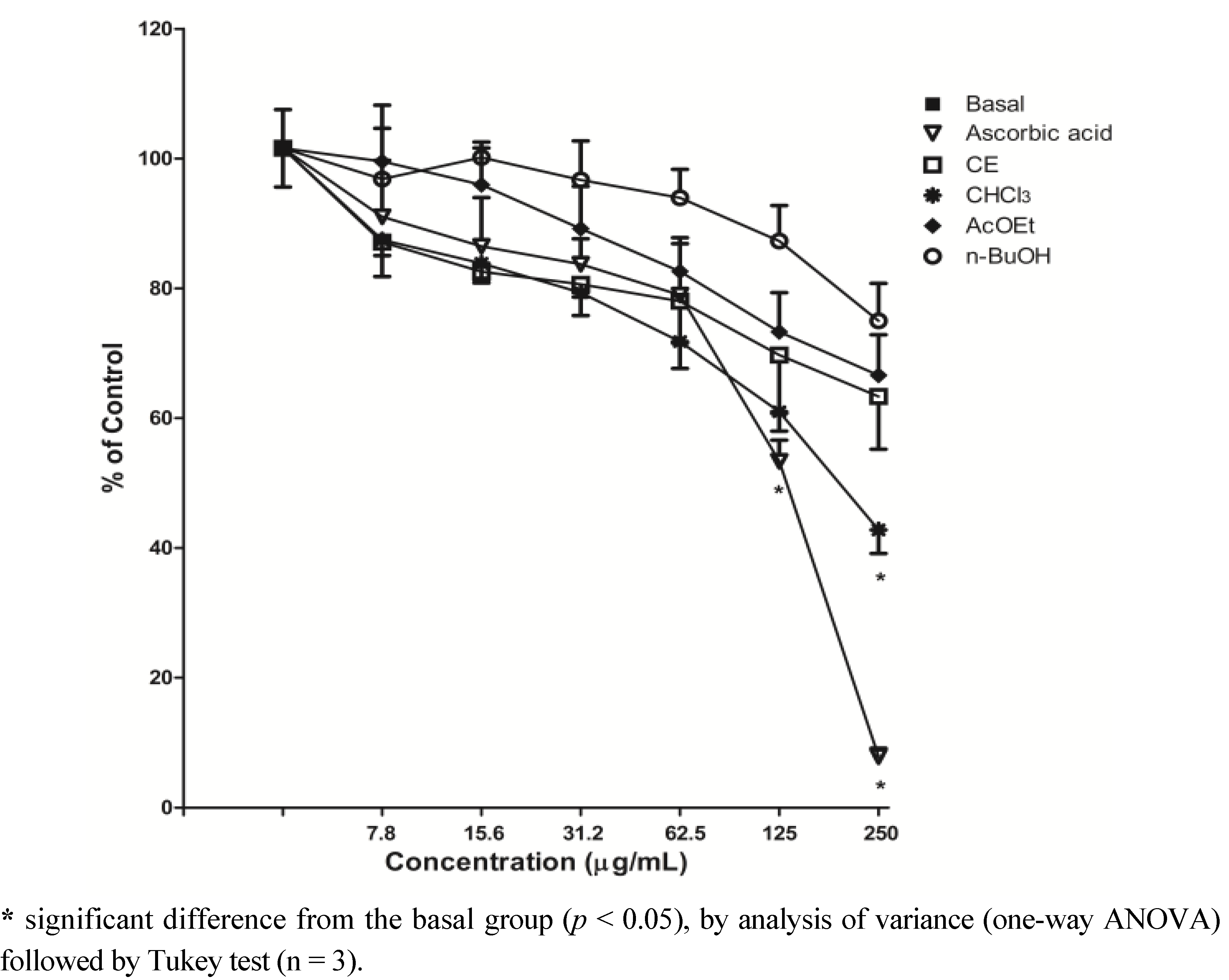
2.4. Inhibition of Lipid Peroxidation-TBARS Assay
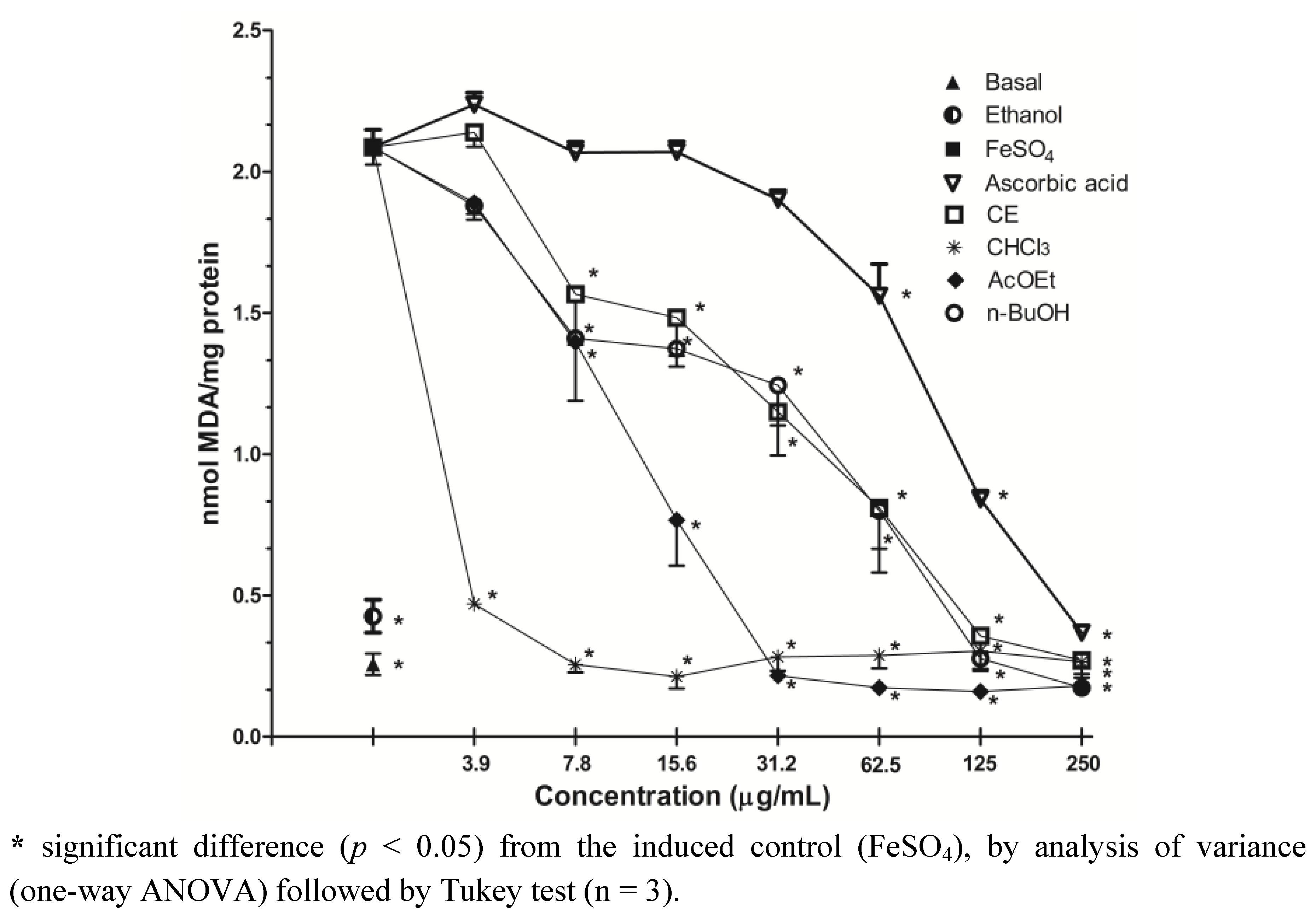
2.5. Inhibition of Protein Oxidation-Carbonyl Content
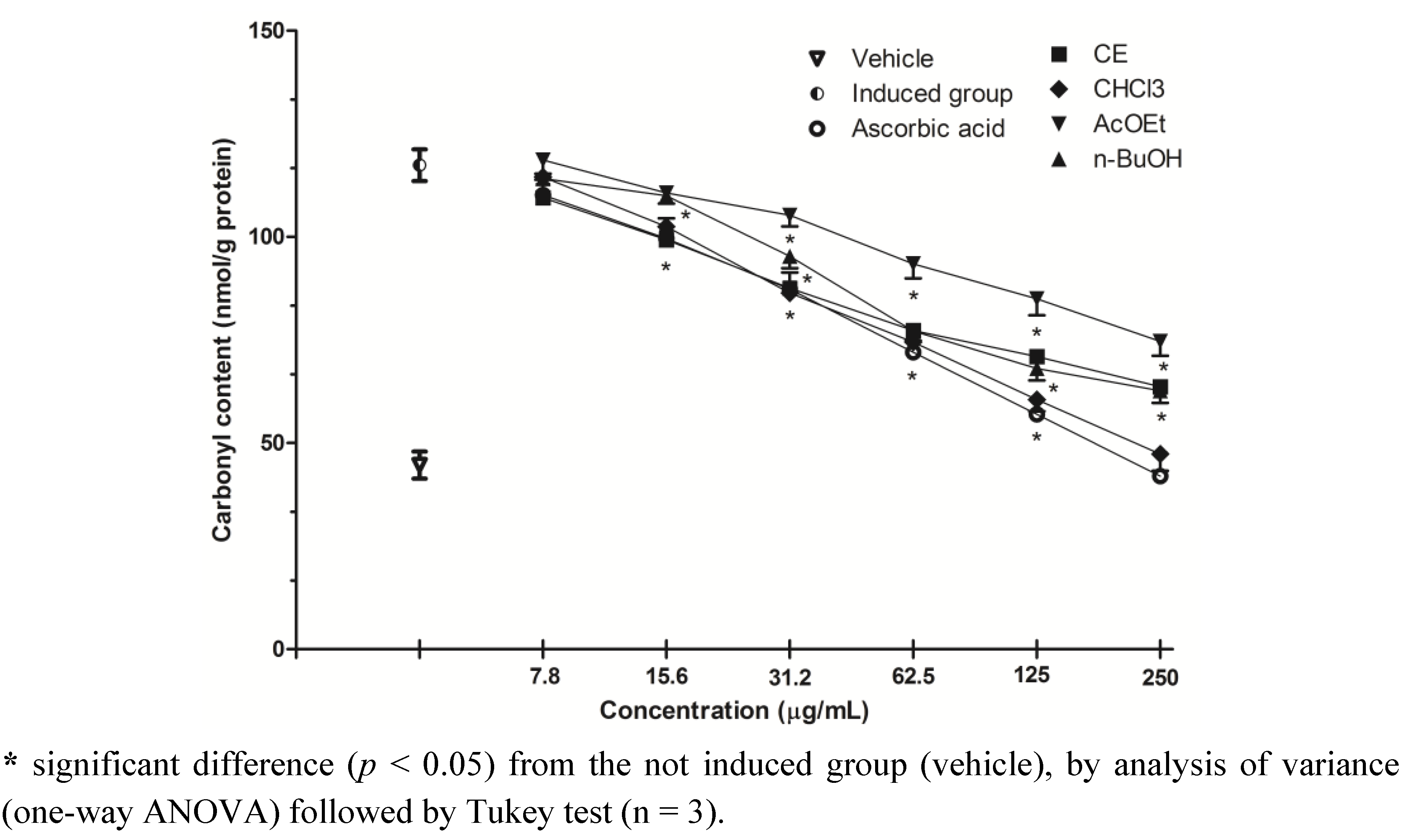
2.6. HPLC/DAD Analysis
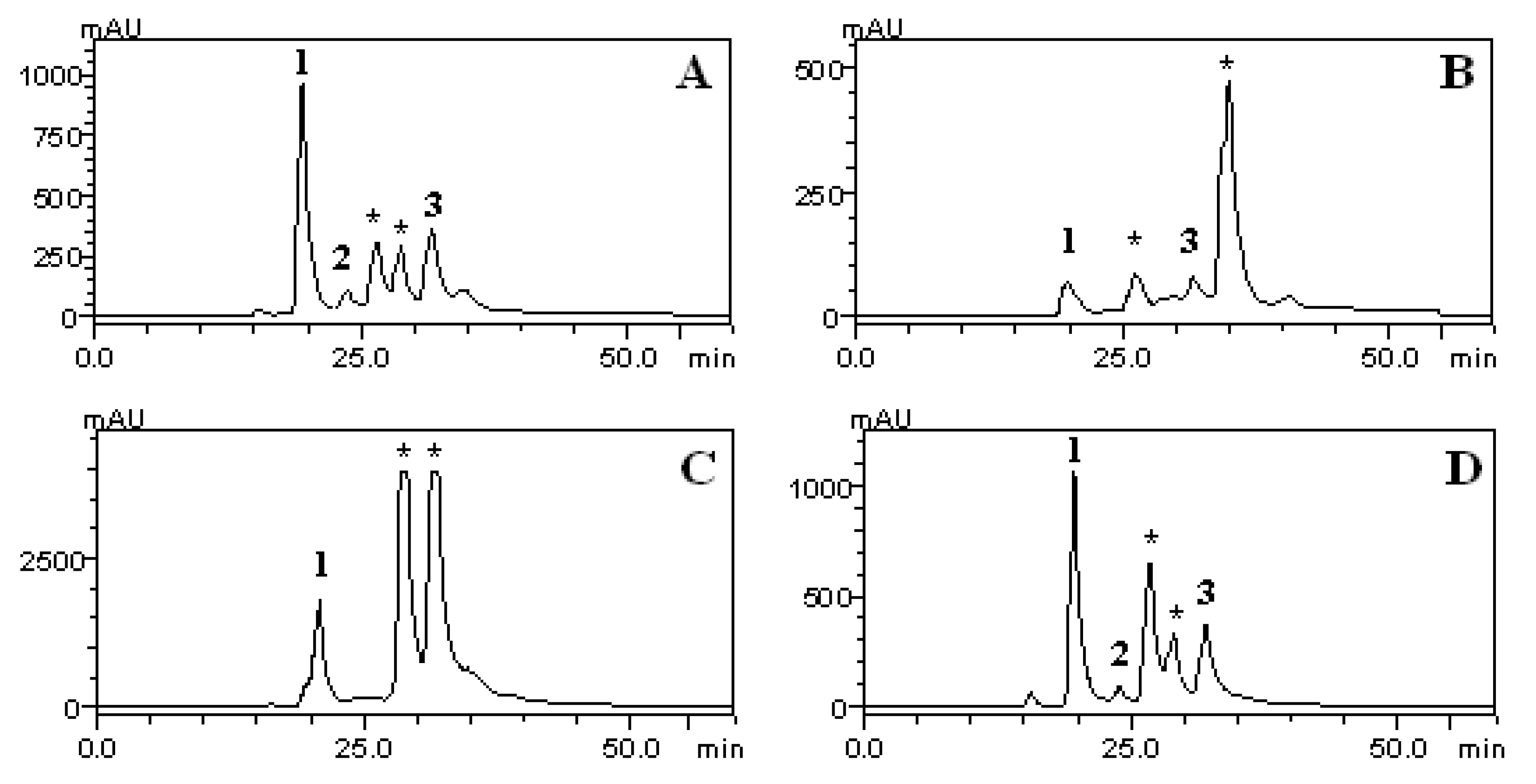
| Extract or fraction | CLA(mg/g) | CFA(mg/g) | RA(mg/g) |
|---|---|---|---|
| CE | 11.15 b ± 0.14 | 1.80 a ± 0.15 | 51.92 a ± 5.58 |
| CHCl3 | 3.23 c ± 1.04 | - | 2.01 b ± 0.1 |
| AcOEt | 21.55 a ± 0.73 | - | - |
| n-BuOH | 11.60 b ± 1.95 | 0.45 b ± 0.2 | 54.79 a ± 4.1 |
3. Experimental
3.1. Chemicals
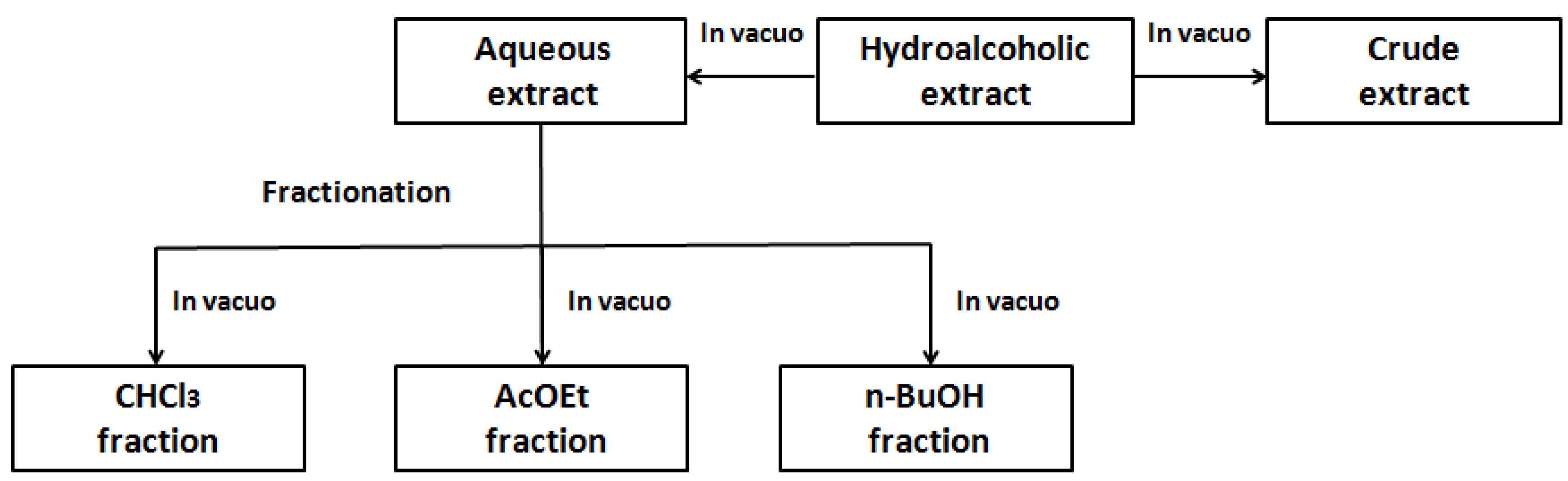
3.2. Plant Collection and Extractions
3.3. Phytochemical Analysis
3.3.1. Total Polyphenols Content
3.3.2. Total Flavonoid Content
3.3.3. Determination of Condensed Tannins
3.3.4. Determination of Total Alkaloids
3.3.5. HPLC/DAD Analysis
3.4. Animals
3.5. Human Serum
3.6. Antioxidant activity Methods
3.6.1. DPPH Radical Scavenging Capacity

3.6.2. DCFH-DA Method
3.6.3. Measurement of Inhibition of Lipid Peroxidation (TBARS Assay)
3.6.4. Measurement of Inhibition of Protein Oxidation (Carbonyl assay)
3.7. Statistical Analysis
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds are available from the authors.
References
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Yang, J.; Martinson, T.E.; Liu, R.H. Phytochemical profiles and antioxidant activities of wine grapes. Food Chem. 2009, 116, 332–339. [Google Scholar] [CrossRef]
- Erkan, N. Antioxidant activity and phenolic compounds of fractions from Portulaca oleracea L. Food Chem. 2012, 133, 775–781. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Mayne, S.T. Antioxidant nutrients and chronic disease: Use of biomarkers of exposure and oxidative stress status in epidemiologic research. J. Nutr. 2003, 133, 933–940. [Google Scholar]
- Boligon, A.A.; Pereira, R.P.; Feltrin, A.C.; Machado, M.M.; Janovik, V.; Rocha, J.B.T.; Athayde, M.L. Antioxidant activities of flavonol derivates from the leaves and stem bark of Scutia buxifolia Reiss. Bioresource Technol. 2009, 100, 6592–6598. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Costa, J.G.M.; Leite, G.O.; Dubois, A.F.; Seeger, R.L.; Boligon, A.A.; Athayde, M.L.; Campos, A.R.; Rocha, J.B.T. Antioxidant effect of Stryphnodendron rotundifolium Martius extracts from Cariri-Ceará state (Brazil): Potential involvement in its therapeutic use. Molecules 2012, 17, 934–950. [Google Scholar]
- Moon, J.K.; Shibamoto, T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef]
- Oliveira, R.C.M.; Monteiro, F.S.; Silva, J.L.V.; Ribeiro, L.A.A.; Santos, R.F.; Nascimento, R.J.B.; Duarte, J.C.; Agra, M.F.; Silva, T.M.S.; Almeida, F.R.C.; et al. Methanol and ethyl acetate extracts from Solanum megalonyx Sendtn. (Solanaceae) present spasmolytic activity in guinea-pig ileum: A comparative study. Rev. Bras. Farmacogn. 2006, 16, 146–151. [Google Scholar]
- Silva, T.M.S.; Carvalho, M.G.; Braz-Filho, R.; Agra, M.F. Occurrence of flavones and flavonols aglycones and its glycosides in Solanum (Solanaceae). Quim. Nova 2003, 26, 517–522. [Google Scholar] [CrossRef]
- Soares, E.L.C.; Vignoli-Silva, M.; Vendruscolo, G.S.; Thode, V.A.; Silva, J.G.; Mentz, L.A. Solanaceae in the Parque Estadual de Itapuã, Viamão, Rio Grande do Sul, Brasil. R. Bras. Bioci. 2008, 6, 177–188. [Google Scholar]
- Costa, O.A. Jurubeba. Rev. Bras. Farm. 1940, 21, 404–416. [Google Scholar]
- Simões, C.M.O.; Falkenberg, M.; Mentz, L.A.; Schenkel, E.P.; Amoros, M.; Girre, L. Antiviral activity of south Brazilian medicinal plant extracts. Phytomedicine 1999, 6, 205–214. [Google Scholar] [CrossRef]
- Sabir, S.M.; Rocha, J.B.T. Antioxidant and hepatoprotective activity of aqueous extract of Solanum fastigiatum (false “Jurubeba”) against paracetamol-induced liver damage in mice. J. Ethnopharmacol. 2008, 120, 226–232. [Google Scholar] [CrossRef]
- Janovik, V.; Boligon, A.A.; Bandeira, R.V.; Athayde, M.L. HPLC/DAD analysis, determination of total phenolic and flavonoid contents and antioxidant activity from the leaves of Cariniana domestica (Mart) Miers. Res. J. Phytochem. 2011, 5, 209–215. [Google Scholar] [CrossRef]
- Schubert, A.; Pereira, D.F.; Zanin, F.F.; Alves, S.H.; Beck, R.C.R.; Athayde, M.L. Comparison of antioxidant activities and total polyphenolic and methylxanthine contents between the unripe fruit and leaves of Ilex paraguariensis A. St. Hil. Pharmazie 2007, 62, 876–880. [Google Scholar]
- Silva, D.A.; Silva, T.M.S.; Lins, A.C.S.; Costa, D.A.; Cavalcante, J.M.S.; Matias, W.N.; Souza, M.F.V.; Filho, B.R. Chemical constituents and antioxidant activity of Sida galheirensis Ulbr. (Malvaceae). Quim. Nova 2006, 29, 1250–1254. [Google Scholar]
- Rota, C.; Chignell, C.F.; Mason, R.P. Evidence for free radical formation during the oxidation of 2′-7′-dichlorofluorescin to the fluorescent dye 2′-7′-dichlorofluorescein by horseradish peroxidase: Possible implications for oxidative stress measurements. Free Radic. Biol. Med. 1999, 27, 873–881. [Google Scholar] [CrossRef]
- He, K.; Li, X.; Ye, X.; Yuan, L.; Li, X.; Chen, X.; Deng, Y. A mitochondria-based method for the determination of antioxidant activities using 2′,7′-dichlorofluorescin diacetate oxidation. Food Res. Int. 2012, 48, 454–461. [Google Scholar] [CrossRef]
- Liang, C.H.; Chan, L.P.; Ding, H.Y.; So, E.C.; Lin, R.J.; Wang, H.M.; Chen, Y.G.; Chou, T.H. Free radical scavenging activity of 4-(3,4-dihydroxybenzoyloxymethyl)phenyl-O-β-D-glucopyranoside from Origanum vulgare and its protection against oxidative damage. J. Agric. Food Chem. 2012, 60, 7690–7696. [Google Scholar]
- Sini, H.; Devi, K.S. Antioxidant activities of the chloroform extract of Solanum trilobatum. Pharm. Biol. 2004, 42, 462–466. [Google Scholar] [CrossRef]
- Dalle-Done, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. Methods to measure the antioxidant activity in plant material. A comparative discussion. Free Radic. Res. 1999, 31, 89–96. [Google Scholar] [CrossRef]
- Ndhlala, A.R.; Moyo, M.; Staden, J.V. Natural antioxidants: Fascinating or mythical biomolecules? Molecules 2010, 15, 6905–6930. [Google Scholar]
- Palafox-Carlos, H.; Yahia, E.M.; Gonzáles-Aguilar, G.A. Identification and quantification of major phenolic compounds (Mangifera indica, cv. Ataulfo) fruit by HPLC-DAD-MS/MS-ESI and their individual contribution to the antioxidant activity during ripening. Food Chem. 2012, 135, 105–111. [Google Scholar]
- Li, G.S.; Jiang, W.L.; Tian, J.W.; Qu, G.W.; Zhu, H.B.; Fu, F.H. In vitro and in vivo effects of rosmarinic acid on experimental liver fibrosis. Phytomedicine 2010, 17, 282–288. [Google Scholar] [CrossRef]
- Lin, J.Y.; Chen, Y.C.; Lee, Y.C.; Hou, C.W.R.; Chen, F.L.; Yang, D.J. Antioxidant, anti-proliferative and cyclooxigenase-2 inhibitory activities of ethanolic extracts from lemon balm (Melissa officinalis L.) leaves. LWT-Food Sci. Technol. 2012, 49, 1–7. [Google Scholar]
- Chandra, S.; Mejia, E.G. Polyphenolic compounds, antioxidant capacity, and quinine reductase activity of an aqueous extract of Ardisia compressa in comparison to mate (Ilex paraguariensis) and green (Camellia sinensis) teas. J. Agric. Food Chem. 2004, 52, 3583–3589. [Google Scholar]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apicult. Res. 1998, 37, 99–105. [Google Scholar]
- Morrison, I.M.; Asiedu, E.A.; Stuchbury, T.; Powell, A.A. Determination of lignin and tannin contents of Cowpea seed coats. Ann. Bot. Lond. 1995, 76, 287–290. [Google Scholar] [CrossRef]
- Sreevidya, N.; Mehrotra, S. Spectrophotometric method for estimation of alkaloids precipitable with Dragendorff’s reagent in plant materials. J. AOAC Int. 2003, 86, 1124–1127. [Google Scholar]
- Evaristo, I.M.; Leitão, M.C. Identification and quantification by HPLC-DAD, of the phenolic fraction contained in leaves of Quercus suber L. Silva Lusitana 2001, 9, 135–141. [Google Scholar]
- Choi, C.W.; Kim, S.C.; Hwang, S.S.; Choi, B.K.; Ahn, H.J.; Lee, M.Y.; Park, S.H.; Kim, S.K. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci. 2002, 163, 1161–1168. [Google Scholar] [CrossRef]
- Myrhe, O.; Andersen, J.M.; Aarnes, H.; Fonnum, F. Evaluation of the probes 2′,7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem. Pharmacol. 2003, 65, 1575–1582. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar]
- Morabito, F.; Cristani, M.; Saija, A.; Stelitano, C.; Callea, V.; Tomaino, A.; Minciullo, P.L.; Gangemi, S. Lipid peroxidation and protein oxidation in patients affected by Hodgkin’s lymphoma. Mediat. Inflamm. 2004, 13, 381–383. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zadra, M.; Piana, M.; Brum, T.F.d.; Boligon, A.A.; Freitas, R.B.d.; Machado, M.M.; Stefanello, S.T.; Soares, F.A.A.; Athayde, M.L. Antioxidant Activity and Phytochemical Composition of the Leaves of Solanum guaraniticum A. St.-Hil. Molecules 2012, 17, 12560-12574. https://doi.org/10.3390/molecules171112560
Zadra M, Piana M, Brum TFd, Boligon AA, Freitas RBd, Machado MM, Stefanello ST, Soares FAA, Athayde ML. Antioxidant Activity and Phytochemical Composition of the Leaves of Solanum guaraniticum A. St.-Hil. Molecules. 2012; 17(11):12560-12574. https://doi.org/10.3390/molecules171112560
Chicago/Turabian StyleZadra, Marina, Mariana Piana, Thiele Faccim de Brum, Aline Augusti Boligon, Robson Borba de Freitas, Michel Mansur Machado, Sílvio Terra Stefanello, Félix Alexandre Antunes Soares, and Margareth Linde Athayde. 2012. "Antioxidant Activity and Phytochemical Composition of the Leaves of Solanum guaraniticum A. St.-Hil" Molecules 17, no. 11: 12560-12574. https://doi.org/10.3390/molecules171112560




