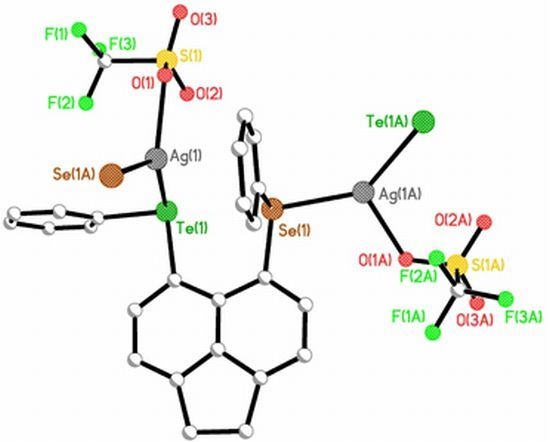
Investigating Silver Coordination to Mixed Chalcogen Ligands
Abstract
:1. Introduction
2. Results and Discussion
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| Peri-atoms | Se, S | Te, S | Te, Se | Se, S | Te, S | Te, Se |
| 77Se-NMR | 379.1 | - | 322.6 | 369.9 | - | 321.6 |
| 125Te-NMR | - | 567.0 | 544.1 | - | 552.3 | 537.2 |
| L1 | L2 | L3 | ||||
| 77Se-NMR | 433.7 | - | 340.7 | |||
| 125Te-NMR | - | 689.4 | 663.4 |
| Compound | 1 | 2 | 3 | 4 | 5 | 6 | ||
|---|---|---|---|---|---|---|---|---|
| Ligand; peri-atoms | L1; Se,S | L1; Se,S | L2; TeS | L2; TeS | L3; TeSe | L1; Se,S | L2; TeS | L3; TeSe |
| E···E' | 3.1122(15) | 3.1018(16) | 3.1502(16) | 3.1581(16) | 3.2342(18) | 3.14(4) | 3.1669(10) | 3.2417(11) |
| ΣrvdW—E···E' [a]; % ΣrvdW [a] | 0.588; 84 | 0.598; 84 | 0.710; 82 | 0.702; 82 | 0.726; 82 | 0.460; 87 | 0.693; 82 | 0.718; 82 |
| Peri-region bond angles | ||||||||
| E(1)-C(1)-C(10) | 122.1(4) | 122.1(4) | 122.4(4) | 123.9(4) | 124.9(7) | 122(8) | 122.6(2) | 124.7(6) |
| C(1)-C(10)-C(9) | 129.3(5) | 129.3(5) | 131.1(5) | 129.0(5) | 128.4(10) | 130(12) | 130.1(3) | 130.2(7) |
| E'(1)-C(9)-C(10) | 123.0(4) | 122.8(5) | 121.1(5) | 121.4(4) | 125.0(9) | 124(12) | 121.7(3) | 121.6(6) |
| Σ of bay angles | 374.4(11) | 374.2(11) | 374.6(11) | 374.3(11) | 378.3(20) | 376(24) | 374.4(6) | 376.5(16) |
| Splay angle [b] | 14.4 | 14.2 | 14.6 | 14.3 | 18.3 | 16.0 | 14.4 | 16.5 |
| Out-of-plane displacement | ||||||||
| E | −0.298(1) | −0.289(1) | −0.332(1) | −0.307(1) | −0.267(1) | 0.375(1) | 0.400(1) | 0.173(1) |
| 0.177(1) | 0.208(1) | 0.150(1) | 0.189(1) | 0.143(1) | −0.183(1) | −0.114(1) | −0.393(1) | |
| Central naphthalene ring torsion angles | ||||||||
| C:(6)-(5)-(10)-(1) | 177.47(1) | 177.67(1) | 179.19(1) | 177.11(1) | 177.83(1) | 179.27(1) | 178.65(1) | 178.77(1) |
| C:(4)-(5)-(10)-(9) | 177.97(1) | 178.20(1) | 177.01(1) | 178.65(1) | 179.58(1) | 175.92(1) | 178.07(1) | 176.60(1) |
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| Ag1-E1 | 2.6252(7) | 2.7053(9) | 2.7023(12) | 2.628(14) | 2.7304(8) | |
| Ag1-E11 | 2.628(14) | 2.7304(8) | ||||
| Ag1-E12 | 2.628(14) | 2.7304(8) | ||||
| Ag1-E2 | 2.6236(7) | 2.7066(9) | 2.7023(12) | |||
| Ag1-cg(19-24) | 3.002(1) | 3.065(1) | 3.119(1) | |||
| Ag1-cg(49-54) | 2.982(1) | 3.054(1) | 3.119(1) | |||
| Ag1···Ag11 | 8.481(1) | 8.515(1) | 8.856(1) | 9.468(1) | 9.728(1) | |
| E1-Ag1-E11 | 119.5(4) | 119.977(6) | ||||
| E1-Ag1-E12 | 119.5(4) | 119.977(6) | ||||
| E11-Ag1-E12 | 119.5(4) | 119.977(6) | ||||
| E1-Ag1-E2 | 135.14(3) | 140.40(3) | 144.55(6) | |||
| E1-Ag1-cg(19-24) | 105.67(1) | 104.67(1) | 106.65(1) | |||
| E1-Ag1-cg(49-54) | 89.80(1) | 88.57(1) | 87.74(1) | |||
| E2-Ag1-cg(19-24) | 89.43(1) | 88.13(1) | 106.65(1) | |||
| E2-Ag1-cg(49-54) | 105.90(1) | 104.83(1) | 87.74(1) | |||
| cg(19-24)-Ag1-cg(49-54) | 139.32(1) | 141.06(1) | 132.09(1) | |||
| Ag1-Te1 | 2.6807(11) | |||||
| Ag1-Se11 | 2.6013(12) | |||||
| Ag1-O1 | 2.360(6) | |||||
| Ag1···Ag1 | 5.929(1) | |||||
| Te1-Ag1-Se1 | 132.70(4) | |||||
| Te1-Ag1-O1 | 111.72(15) | |||||
| Se1-Ag1-O1 | 112.75(15) |
| 1 | 2 | 3 | |
|---|---|---|---|
| Empirical Formula | C48H36AgBF4S2Se2·2CH2Cl4 | C48H36AgBF4S2Te2·2CH2Cl4 | C48H36AgBF4Se2Te2·CH2Cl2 |
| Formula Weight | 1199.39 | 1296.67 | 1305.54 |
| Temperature (°C) | −180(1) | −180(1) | −180(1) |
| Crystal Colour, Habit | colourless, prism | colourless, platelet | colourless, platelet |
| Crystal Dimensions (mm3) | 0.100 × 0.100 × 0.020 | 0.100 × 0.100 × 0.010 | 0.080 × 0.060 × 0.020 |
| Crystal System | monoclinic | monoclinic | monoclinic |
| Lattice Parameters | a = 11.783(3) Å | a = 11.823(5) Å | a = 11.923(6) Å |
| b = 28.585(5) Å | b = 29.132(9) Å | b = 29.68(2) Å | |
| c = 14.459(3) Å | c = 14.588(5) Å | c = 14.794(7) Å | |
| - | - | - | |
| β = 106.853(5)° | β = 107.227(9)° | β = 105.833(11)° | |
| - | - | - | |
| Volume (Å3) | V = 4661(2) | V = 4799(3) | V = 5037(4) |
| Space Group | P21/n | P21/n | C2/c |
| Z value | 4 | 4 | 4 |
| Dcalc (g/cm3) | 1.709 | 1.795 | 1.721 |
| F000 | 2384 | 2528 | 2504 |
| μ(MoKα) (cm−1) | 23.646 | 19.714 | 31.322 |
| No. of Reflections Measured | 28463 | 25411 | 15364 |
| Rint | 0.0409 | 0.0691 | 0.1069 |
| Min and Max Transmissions | 0.820–0.954 | 0.633–0.980 | 0.476–0.939 |
| Observed Reflection (No. Variables) | 8467(577) | 8419(577) | 4395(285) |
| Reflection/Parameter Ratio | 14.67 | 14.59 | 15.42 |
| Residuals: R1 (I > 2.00σ(I)) | 0.0521 | 0.0703 | 0.0786 |
| Residuals: R (All reflections) | 0.0712 | 0.1029 | 0.1527 |
| Residuals: wR2 (All reflections) | 0.1603 | 0.2006 | 0.2629 |
| Goodness of Fit Indicator | 1.081 | 1.083 | 1.029 |
| Maximum peak in Final Diff. Map | 1.93 e−/Å3 | 2.39 e−/Å3 | 1.21 e−/Å3 |
| Minimum peak in Final Diff. Map | −0.98 e−/Å3 | −1.82 e−/Å3 | −0.75 e−/Å3 |
| 4 | 5 | 6 | |
|---|---|---|---|
| Empirical Formula | C73H54AgF3O3S4Se3 | C73H54AgF3OS4Te3 | C25H18AgF3O3SSeTe·CH2Cl2 |
| Formula Weight | 1509.21 | 1623.13 | 854.83 |
| Temperature (°C) | −180(1) | −180(1) | −180(1) |
| Crystal Colour, Habit | colourless, prism | yellow, prism | colourless, prism |
| Crystal Dimensions (mm3) | 0.200 × 0.100 × 0.100 | 0.050 × 0.050 × 0.050 | 0.030 × 0.030 × 0.030 |
| Crystal System | trigonal | trigonal | monoclinic |
| Lattice Parameters | a = 18.314(4) Å | a = 18.369(5) Å | a = 15.135(5) Å |
| - | - | b = 10.141(3) Å | |
| c = 33.789(8) Å | c = 34.233(8) Å | c = 17.970(5) Å | |
| - | - | - | |
| - | - | β = 92.633(8)° | |
| - | - | - | |
| Volume (Å3) | V = 9814(4) | V = 10004(5) | V = 2755(2) |
| Space Group | R-3 | R-3 | P21/n |
| Z value | 6 | 6 | 4 |
| Dcalc (g/cm3) | 1.532 | 1.616 | 2.061 |
| F000 | 4536 | 4764 | 1640 |
| μ (MoKα) (cm−1) | 21.601 | 17.643 | 34.070 |
| No. of Reflections Measured | 20776 | 21511 | 17001 |
| Rint | 0.0522 | 0.0591 | 0.0700 |
| Min and Max Transmissions | 0.507–0.806 | 0.636–0.916 | 0.531–0.903 |
| Observed Reflection (No. Variables) | 3974(310) | 4060(283) | 5024(343) |
| Reflection/Parameter Ratio | 12.82 | 14.35 | 14.65 |
| Residuals: R1 (I > 2.00σ(I)) | 0.0797 | 0.0717 | 0.0535 |
| Residuals: R (All reflections) | 0.0965 | 0.0877 | 0.0678 |
| Residuals: wR2 (All reflections) | 0.2427 | 0.2092 | 0.1513 |
| Goodness of Fit Indicator | 1.061 | 1.200 | 1.102 |
| Maximum peak in Final Diff. Map | 1.88 e−/Å3 | 1.70 e−/Å3 | 1.49 e−/Å3 |
| Minimum peak in Final Diff. Map | −0.84 e−/Å3 | −1.70 e−/Å3 | −1.39 e−/Å3 |
| Formula | Donor | Ag(I) | Ligand | Structural Architecture | ||
|---|---|---|---|---|---|---|
| Geometry | coordination | |||||
| 1 | [Ag(L1)2BF4] | SeS | Tetrahedral | Quasi-chelating; | Monomeric, mononuclear, 4-coordinate | |
| 2 | [Ag(L2)2BF4] | TeS | silver(I) sandwich complex containing a | |||
| monodentate, | ||||||
| bent metallocene type fragment and two | ||||||
| 3 | [Ag(L3)2BF4] | TeSe | η6-E(phenyl) | η6- E(phenyl)-Ag interactions | ||
| 4 | [Ag(L1)3CF3SO3] | SeS | Trigonal | Monodentate | Monomeric, mononuclear, 3-coordinate | |
| 5 | [Ag(L2)3CF3SO3] | TeS | planar | silver(I) complex | ||
| 6 | [Ag(L3)CF3SO3]n | TeSe | Trigonal | Bis- monodentate | 1D extended helical chain polymer | |
| planar | μ2-η2-bridging, | containing one left-handed chain | ||||
| [(-Ag-Te-C-C-C-Se-Ag-)n] | ||||||
| 2a | [Ag(L2)3BF4] | TeS | Trigonal | Monodentate | Monomeric, mononuclear, 3-coordinate | |
| 3a | [Ag(L3)3BF4] | TeSe | planar | silver(I) complex |
2.1. Reactions of Silver(I) Tetrafluoroborate
2.1.1. [AgBF4(L1)2] 1, [AgBF4(L2)2] 2 & [AgBF4(L3)2] 3




2.2. Reactions of Silver(I) Trifluoromethanesulfonate
2.2.1. [AgOTf(L1)3] 4 & [AgOTf(L2)3] 5



2.2.2. [AgOTf(L3)]n 6




2.2.3. [AgBF4(L2)3] 2a & [AgBF4(L3)3] 3a
3. Experimental
3.1. General
3.2. Crystal Structure Analyses
4. Conclusions
Supplementary Materials
Acknowledgments
References
- Cotton, F.A.; Wilkinson, G. Advanced Inorganic Chemistry, 5th ed; Wiley: New York, NY, USA, 1988. [Google Scholar]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed; Pergamon: Oxford, UK, 1984. [Google Scholar]
- Shriver, D.F.; Atkins, P.W. Inorganic Chemistry, 3rd ed; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Venkataraman, D.; Du, Y.; Wilson, S.R.; Hirsch, K.A.; Zhang, P.; Moore, J.S. A Coordination Geometry Table of the d-Block Elements and Their Ions. J. Chem. Educ. 1997, 74, 915–918. [Google Scholar] [CrossRef]
- Chen, C.-T.; Suslick, K.S. One-dimensional coordination polymers: Applications to material science. Coord. Chem. Rev. 1993, 128, 293–322. [Google Scholar]
- Fujita, M.; Kwon, Y.J.; Washizu, S.; Ogura, K. Preparation, Clathration Ability, and Catalysis of a Two-Dimensional Square Network Material Composed of Cadmium(II) and 4,4'-Bipyridine. J. Am. Chem. Soc. 1994, 116, 1151–1152. [Google Scholar] [CrossRef]
- Hoskins, B.F.; Robson, R. Design and construction of a new class of scaffolding-like materials comprising infinite polymeric frameworks of 3D-linked molecular rods. A reappraisal of the zinc cyanide and cadmium cyanide structures and the synthesis and structure of the diamond-related frameworks [N(CH3)4][CuIZnII(CN)4] and CuI[4,4',4'',4'''-tetracyanotetraphenylmethane]BF4.xC6H5NO2. J. Am. Chem. Soc. 1990, 112, 1546–1554. [Google Scholar] [CrossRef]
- Carlucci, L.; Ciani, G.; Proserpio, D.M.; Sironi, A. 1-, 2-, and 3-Dimensional polymeric frames in the coordination chemistry of AgBF4 with pyrazine. The first example of three interpenetrating 3-dimensional triconnected nets. J. Am. Chem. Soc. 1995, 117, 4562–4569. [Google Scholar]
- Gardner, G.B.; Venkataraman, D.; Moore, J.S.; Lee, S. Spontaneous assembly of a hinged coordination network. Nature 1995, 374, 792–795. [Google Scholar] [CrossRef]
- Li, B.; Zang, S.-Q.; Liang, R.; Wu, Y.-J.; Mak, T.C.W. Silver(I)-Organic Networks Assembled with Propargyl-Functionalized Di- and Trihydroxybenzenes. Organometallics 2011, 30, 1710–1718. [Google Scholar] [CrossRef]
- Li, J.-R.; Bu, X.-He.; Jiao, J.; Du, W.-P.; Xu, X.-H.; Zhang, R.-H. Novel dithioether-silver(I) coordination architectures: Structural diversities by varying the spacers and terminal groups of ligands. Dalton Trans. 2005, 2005, 464–474. [Google Scholar]
- Burrows, A.D.; Kelly, D.J.; Mahon, M.F.; Raithby, P.R.; Richardson, C.; Stevenson, A.J. Silvercoordination networks and cages based on a semi-rigid bis(isoxazoylyl)ligand. Dalton Trans. 2011, 40, 5483–5493. [Google Scholar] [CrossRef]
- Bu, X.-H.; Chen, W.; Hou, W.-F.; Du, M.; Zhang, R.-H.; Brisse, F. Controlling the Framework Formation of Silver(I) Coordination Polymers with 1,4-Bis(phenylthio)butane by Varying the Solvents, Metal-to-Ligand Ratio, and Counteranions. Inorg. Chem. 2002, 41, 3477–3482. [Google Scholar] [CrossRef]
- Li, J.-R.; Zhang, R.-H.; Bu, X.-H. Synthesis and crystal structure of a 2D (6,3) coordination network, {[Ag(L)1.5]NO3}n [L = 1,3-bis(benzylthio)propane]. J. Chem. Crystallogr. 2004, 34, 501–505. [Google Scholar] [CrossRef]
- Booth, D.G.; Levason, W.; Quirk, J.J.; Reid, G.; Smith, S.M. Synthesis and characterisation of transition-metal complexes involving cyclic diselenoether ligands. Dalton Trans. 1997, 1997, 3493–3500. [Google Scholar]
- Young, A.G.; Hanton, L.R. Square planar silver(I) complexes: A rare but increasingly observed stereochemistry for silver(I). Coord. Chem. Rev. 2008, 252, 1346–1386. [Google Scholar] [CrossRef]
- Tan, C.-K.; Wang, J.; Leng, J.-D.; Zheng, L.-L.; Tong, M.-L. The Use of 2,1,3-Benzoselenadiazole as an Auxiliary Ligand for the Construction of New 2D Silver(I)/Benzene- or Cyclohexane-1,3,5-tricarboxylate Honeycomb Networks. Eur. J. Inorg. Chem. 2008, 2008, 771–778. [Google Scholar]
- Coulson, C.A.; Daudel, R.; Robertson, J.M. Bond Lengths in Naphthalene and Anthracene. Proc. R. Soc. London Ser. A 1951, 207, 306–320. [Google Scholar] [CrossRef]
- Cruickshank, D.W. A Detailed Refinement of the Crystal and Molecular Structure of Naphthalene. Acta Crystallogr. 1957, 10, 504–508. [Google Scholar] [CrossRef]
- Brock, C.P.; Dunitz, J.D. Temperature Dependence of Thermal Motion in Crystalline Naphthalene. Acta Crystallogr. B 1982, 38, 2218–2228. [Google Scholar] [CrossRef]
- Oddershede, J.; Larsen, S. Charge Density Study of Naphthalene Based on X-ray Diffraction Data at Four Different Temperatures and Theoretical Calculations. J. Phys. Chem. A 2004, 108, 1057–1063. [Google Scholar] [CrossRef]
- Hazell, A.C.; Hazell, R.G.; Norskov-Lauritsen, L.; Briant, C.E.; Jones, D.W. A neutron diffraction study of the crystal and molecular structure of acenaphthene. Acta Crystallogr. C 1986, 42, 690. [Google Scholar] [CrossRef]
- Kilian, P.; Knight, F.R.; Woollins, J.D. Naphthalene and Related Systems peri-Substituted by Group 15 and 16 Elements. Chem. Eur. J. 2011, 17, 2302–2328. [Google Scholar]
- Kilian, P.; Knight, F.R.; Woollins, J.D. Synthesis of ligands based on naphthalene peri-substituted by Group 15 and 16 elements and their coordination chemistry. Coord. Chem. Rev. 2011, 255, 1387–1413. [Google Scholar] [CrossRef]
- Aucott, S.M.; Milton, H.L.; Robertson, S.D.; Slawin, A.M.Z.; Walker, G.D.; Woollins, J.D. Platinum Complexes of Naphthalene-1,8-dichalcogen and Related Polyaromatic Hydrocarbon Ligands. Chem. Eur. J. 2004, 10, 1666–1676. [Google Scholar] [CrossRef]
- Aucott, S.M.; Milton, H.L.; Robertson, S.D.; Slawin, A.M.Z.; Woollins, J.D. Crystal Structures and Molecular Modelling Of 1,8 Chalcogenide Substituted Naphthalenes. Heteroat. Chem. 2004, 15, 531–542. [Google Scholar]
- Aucott, S.M.; Milton, H.L.; Robertson, S.D.; Slawin, A.M.Z.; Woollins, J.D. The preparation and characterisation of bimetallic Iridium(II) complexes containing derivatised bridging naphthalene-1,8-disulfur or 4,5-dithiolato acephenanthrylene ligands. Dalton Trans. 2004, 2004, 3347–3352. [Google Scholar]
- Aucott, S.M.; Kilian, P.; Milton, H.L.; Robertson, S.D.; Slawin, A.M.Z.; Woollins, J.D. Bis(cyclopentadienyl)titanium complexes of naphthalene-1,8-dithiolates, biphenyl 2,2' dithiolates and related ligands. Inorg. Chem. 2005, 44, 2710–2718. [Google Scholar]
- Aucott, S.M.; Kilian, P.; Robertson, S.D.; Slawin, A.M.Z.; Woollins, J.D. Platinum Complexes of Dibenzo[1,2]dithiine and Related Polyaromatic Hydrocarbon Ligands. Chem. Eur. J. 2006, 12, 895–902. [Google Scholar] [CrossRef]
- Aucott, S.M.; Duerden, D.; Li, Y.; Slawin, A.M.Z.; Woollins, J.D. The Preparation and Characterisation of Hetero and Homo-bimetallic Complexes Containing Bridging Naphthalene-1,8-dithiolato Ligands. Chem. Eur. J. 2006, 12, 5495–5504. [Google Scholar] [CrossRef]
- Kilian, P.; Slawin, A.M.Z.; Woollins, J.D. A structural study of 1,8-Bis(dimethyl phosphonito) naphthalene and related crowded chalcogeno derivatives. Dalton Trans. 2003, 2003, 3876–3885. [Google Scholar]
- Kilian, P.; Philp, D.; Slawin, A.M.Z.; Woollins, J.D. Use of Molecular Scaffolding for the Stabilization of an Intramolecular Dative PIII–PV System. Eur. J. Inorg. Chem. 2003, 2003, 249–254. [Google Scholar] [CrossRef]
- Kilian, P.; Slawin, A.M.Z.; Woollins, J.D. Naphthalene-1,8-diyl Bis(Halogenophosphanes): Novel Syntheses and Structures of Useful Synthetic Building Blocks. Chem. Eur. J. 2003, 9, 215–222. [Google Scholar] [CrossRef]
- Kilian, P.; Slawin, A.M.Z.; Woollins, J.D. New Mode of Sterically imposed Hypercoordination. Chem. Commun. 2003, 2003, 1174–1175. [Google Scholar]
- Kilian, P.; Milton, H.L.; Slawin, A.M.Z.; Woollins, J.D. Chlorides, oxochlorides and oxoacids of 1,8-diphospha naphthalene - A system with enforced close P···P interaction. Inorg. Chem. 2004, 43, 2252–2260. [Google Scholar] [CrossRef]
- Kilian, P.; Slawin, A.M.Z.; Woollins, J.D. Phosphonato-phosphinito peri-substituted naphthalenes. Inorg. Chim. Acta 2005, 358, 1719–1723. [Google Scholar] [CrossRef]
- Kilian, P.; Slawin, A.M.Z.; Woollins, J.D. Preparation and structures of 1,2-dihydro-1,2-diphosphaacenaphthenes and rigid backbone stabilised triphosphenium cation. Dalton Trans. 2006, 2006, 2175–2183. [Google Scholar]
- Knight, F.R.; Fuller, A.L.; Bühl, M.; Slawin, A.M.Z.; Woollins, J.D. Synthetic and Structural Studies of 1,8-Chalcogen Naphthalene Derivatives. Chem. Eur. J. 2010, 16, 7503–7516. [Google Scholar] [CrossRef]
- Knight, F.R.; Fuller, A.L.; Bühl, M.; Slawin, A.M.Z.; Woollins, J.D. Synthetic and Structural Studies of 1-Halo-8-(alkylchalcogeno)naphthalene Derivatives. Chem. Eur. J. 2010, 16, 7605–7616. [Google Scholar]
- Knight, F.R.; Fuller, A.L.; Bühl, M.; Slawin, A.M.Z.; Woollins, J.D. Hypervalent Adducts of Chalcogen-Containing peri-Substituted Naphthalenes; Reactions of Sulfur, Selenium, and Tellurium with Dihalogens. Inorg. Chem. 2010, 49, 7577–7596. [Google Scholar] [CrossRef]
- Fuller, A.L.; Knight, F.R.; Slawin, A.M.Z.; Woollins, J.D. 1-Bromo-8-(phenylselenyl)naphthalene. Acta Crystallogr. E 2007, E63, o3855. [Google Scholar]
- Fuller, A.L.; Knight, F.R.; Slawin, A.M.Z.; Woollins, J.D. 1-Bromo-8-(ethylsulfanyl)naphthalene. Acta Crystallogr. E 2007, E63, o3957. [Google Scholar]
- Fuller, A.L.; Knight, F.R.; Slawin, A.M.Z.; Woollins, J.D. 8-Bromonaphthalen-1-amine. Acta Crystallogr. E 2008, E64, o977. [Google Scholar]
- Knight, F.R.; Fuller, A.L.; Slawin, A.M.Z.; Woollins, J.D. Controlling Cu···Cu distances using halides: (8-Phenylthionaphth-1-yl)diphenylphosphine copper halide dimers. Dalton Trans. 2009, 2009, 8476–8478. [Google Scholar]
- Knight, F.R.; Fuller, A.L.; Slawin, A.M.Z.; Woollins, J.D. Preparation and compounds of (8-methoxynaphth-1-yl)diphenylphosphine. Polyhedron 2010, 29, 1849–1853. [Google Scholar] [CrossRef] [Green Version]
- Knight, F.R.; Fuller, A.L.; Slawin, A.M.Z.; Woollins, J.D. Synthesis and structural study of (8-phenylsulfanylnaphth-1-yl)diphenylphosphine metal complexes. Polyhedron 2010, 29, 1956–1963. [Google Scholar] [CrossRef] [Green Version]
- Knight, F.R.; Fuller, A.L.; Bühl, M.; Slawin, A.M.Z.; Woollins, J.D. Sterically Crowded peri-Substituted Naphthalene Phosphines and their PV Derivatives. Chem. Eur. J. 2010, 16, 7617–7634. [Google Scholar] [CrossRef]
- Fuller, A.L.; Knight, F.R.; Slawin, A.M.Z.; Woollins, J.D. Platinum Complexes of Aromatic Selenolates. Eur. J. Inorg. Chem. 2010, 2010, 4034–4043. [Google Scholar] [CrossRef]
- Aschenbach, L.K.; Knight, F.R.; Randall, R.A.M.; Cordes, D.B.; Baggott, A.; Bühl, M.; Slawin, A.M.Z.; Woollins, J.D. Onset of three-centre, four-electron bonding in peri-substituted acenaphthenes: A structural and computational investigation. Dalton Trans. 2012, 41, 3141–3153. [Google Scholar]
- Knight, F.R.; Athukorala Arachchige, K.S.; Randall, R.A.M.; Bühl, M.; Slawin, A.M.Z.; Woollins, J.D. Exploring hypervalency and three-centre, four-electron bonding interactions: Reactions of acenaphthene chalcogen donors and dihalogen acceptors. Dalton Trans. 2012, 41, 3154–3165. [Google Scholar] [CrossRef]
- Knight, F.R.; Randall, R.A.M.; Athukorala Arachchige, K.S.; Wakefield, L.; Griffin, J.M.; Ashbrook, S.E.; Bühl, M.; Slawin, A.M.Z.; Woollins, J.D. Noncovalent Interactions in Peri-Substituted Chalconium Acenaphthene and Naphthalene Salts: A Combined Experimental, Crystallographic, Computational, and Solid-State NMR Study. Inorg. Chem. 2012, 51, 11087–11097. [Google Scholar]
- Lechner, M.-L.; Athukorala Arachchige, K.S.; Randall, R.A.M.; Knight, F.R.; Bühl, M.; Slawin, A.M.Z.; Woollins, J.D. Sterically Crowded Tin Acenaphthenes. Organometallics 2012, 31, 2922–2930. [Google Scholar] [CrossRef]
- Knight, F.R.; Randall, R.M.; Wakefield, L.; Slawin, A.M.Z.; Woollins, J.D. Silver(I) coordination complexes and extended networks assembled from S, Se, Te substituted acenaphthenes. Dalton Trans. 2013. [Google Scholar] [CrossRef]
- Knight, F.R.; Randall, R.M.; Wakefield, L.; Slawin, A.M.Z.; Woollins, J.D. University of St Andrews, St Andrews, Fife, UK. Unpublished work, 2012. [Google Scholar]
- Bondi, A. Van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Nakanishi, W.; Hayashi, S.; Toyota, S. Structure of bis[8-(phenylselanyl)naphthyl] diselenide: First linear alignment of four Se atoms as a four-centre six-electron bond. Chem. Commun. 1996, 1996, 371–372. [Google Scholar]
- Nakanishi, W.; Hayashi, S.; Sakaue, A.; Ono, G.; Kawada, Y. Attraxtive Interaction Caused by the Linear F···Se-C Alignment in Naphthalene Peri Positions. J. Am. Chem. Soc. 1998, 120, 3635–3640. [Google Scholar] [CrossRef]
- Nakanishi, W.; Hayashi, S.; Toyota, S. Four-Center Six-Electron Interaction versus Lone Pair-Lone Pair Interaction between Selenium Atoms in Naphthalene Peri Positions. J. Org. Chem. 1998, 63, 8790–8800. [Google Scholar] [CrossRef]
- Hayashi, S.; Nakanishi, W. Novel Substituent Effect on 77Se-NMR Chemical Shifts Caused by 4c-6e versus 2c-4e and 3c-4e in Naphthalene Peri Positions: Spectroscopic and Theoretical Study. J. Org. Chem. 1999, 64, 6688–6696. [Google Scholar] [CrossRef]
- Nakanishi, W.; Hayashi, S.; Uehara, T. Successive Change in Conformation Caused by p-Y Groups in 1-(MeSe)-8-(p-YC6H4Se)C10H6: Role of Linear Se···Se-C Three-Center-Four-Electron versus n(Se)···n(Se) Two-Center-Four-Electron Nonbonded Interactions. J. Phys. Chem. A 1999, 103, 9906–9912. [Google Scholar] [CrossRef]
- Nakanishi, W.; Hayashi, S.; Uehara, T. Structure of 1-(Arylselanyl)naphthalenes—Y Dependence in 1-(p-YC6H4Se)C10H7. Eur. J. Org. Chem. 2001, 2001, 3933–3943. [Google Scholar] [CrossRef]
- Nakanishi, W.; Hayashi, S. Nonbonded P···P and P···Se Interactions in Naphthalene 1,8-Positions: Role of Lone-Pair Orbitals. Phosphorus Sulfur Silicon Relat. Elem. 2002, 177, 1833–1837. [Google Scholar] [CrossRef]
- Nakanishi, W.; Hayashi, S.; Arai, T. Linear alignment of four sulphur atoms in bis[(8-phenylthio)naphthyl] disulfude: Contribution of linear S4 hypervalent four-centre six-electron bond to the structure. Chem. Commun. 2002, 2002, 2416–2417. [Google Scholar]
- Hayashi, S.; Nakanishi, W. Structure of 1-(Arylselanyl)naphthalenes. 2. G Dependence in 8-G-1-(p-YC6H4Se)C10H6. J. Org. Chem. 2002, 67, 38–48. [Google Scholar] [CrossRef]
- Nakanishi, W.; Hayashi, S.; Itoh, N. First linear alignment of five C-Se···O···Se-C atoms in anthraquinone and 9-(methoxy)anthracene bearing phenylselanyl groups at 1,8-positions. Chem. Commun. 2003, 2003, 124–125. [Google Scholar]
- Hayashi, S.; Wada, H.; Ueno, T.; Nakanishi, W. Structures of 1-(Arylseleninyl)naphthalenes: O, G, and Y Dependences in 8-G-1-[p-YC6H4Se(O)]C10H6. J. Org. Chem. 2006, 71, 5574–5585. [Google Scholar]
- Hayashi, S.; Nakanishi, W. Noncovalent Z···Z (Z = O, S, Se and Te) Interactions: How Do They Operate to Control Fine Structures of 1,8-Dichalcogene-Substituted Naphthalenes. Bull. Chem. Soc. Jpn. 2008, 81, 1605–1615. [Google Scholar] [CrossRef]
- Nagy, P.; Szabó, D.; Kapovits, I.; Kucsman, Á.; Argay, G.; Kálmán, A. Intramolecular S⋯S and S⋯O close contacts in 1,8-bis(phenylsulfanyl)naphthalene derivatives of different sulfur valence state: An X-ray study. J. Mol. Struct. 2002, 606, 61–76. [Google Scholar] [CrossRef]
- Nishio, M. CH/π hydrogen bonds in crystals. CrystEngComm. 2004, 6, 130–158. [Google Scholar] [CrossRef]
- Fischer, C.; Gruber, T.; Seichter, W.; Schindler, D.; Weber, E. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetramethoxycalix[4]arene dichloromethane hemisolvate. Acta Crystallogr. Sec. E 2008, E64, o673. [Google Scholar]
- Hirota, M.; Sakaibara, K.; Suezawa, H.; Yuzuri, T.; Ankai, E.; Nishio, M. Intramolecular CH-π interaction. Substituent effect as a probe for hydrogen bond-like character. J. Phys. Org. Chem. 2000, 13, 620–623. [Google Scholar]
- Tsubaki, H.; Tohyama, S.; Koike, K.; Saitoh, H.; Ishitani, O. Effect of intramolecular π-π and CH–π interactions between ligands on structure, electrochemical and spectroscopic properties of fac-[Re(bpy)(CO)3(PR3)]+ (bpy = 2,2'-bipyridine; PR3 = trialkyl or triarylphosphines). Dalton Trans. 2005, 2005, 385–395. [Google Scholar]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. D 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Rigaku Corporation, 2010. CrystalClear Software User’s Guide, Molecular Structure Corporation, © 2000. Flugrath, J.W.P. The finer things in X-ray diffraction data collection. Acta Crystallogr. D, 1999; D55, 1718–1725.
- Altomare, A.; Burla, M.; Camalli, M.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Beurskens, P.T.; Admiraal, G.; Beurskens, G.; Bosman, W.P.; de Gelder, R.; Israel, R.; Smits, J.M.M. The DIRDIF-99 Program System; Technical Report of the Crystallography Laboratory, University of Nijmegen: Nijmegen, The Netherlands, 1999; DIRDIF99. [Google Scholar]
- CrystalStructure 4.0, Crystal Structure Analysis Package, Rigaku and Rigaku/MSC (2009). 9009 New Trails Dr., The Woodlands, TX 77381, USA.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Knight, F.R.; Randall, R.A.M.; Wakefield, L.; Slawin, A.M.Z.; Woollins, J.D. Investigating Silver Coordination to Mixed Chalcogen Ligands. Molecules 2012, 17, 13307-13329. https://doi.org/10.3390/molecules171113307
Knight FR, Randall RAM, Wakefield L, Slawin AMZ, Woollins JD. Investigating Silver Coordination to Mixed Chalcogen Ligands. Molecules. 2012; 17(11):13307-13329. https://doi.org/10.3390/molecules171113307
Chicago/Turabian StyleKnight, Fergus R., Rebecca A. M. Randall, Lucy Wakefield, Alexandra M. Z. Slawin, and J. Derek Woollins. 2012. "Investigating Silver Coordination to Mixed Chalcogen Ligands" Molecules 17, no. 11: 13307-13329. https://doi.org/10.3390/molecules171113307
APA StyleKnight, F. R., Randall, R. A. M., Wakefield, L., Slawin, A. M. Z., & Woollins, J. D. (2012). Investigating Silver Coordination to Mixed Chalcogen Ligands. Molecules, 17(11), 13307-13329. https://doi.org/10.3390/molecules171113307