A Comparative Uptake Study of Multiplexed PET Tracers in Mice with Turpentine-Induced Inflammation
Abstract
:1. Introduction
2. Results and Discussion
2.1. PET Tracers
2.2. Biodistribution of Multiple Tracers
2.3. Inflammation PET Imaging with Multiple Tracers
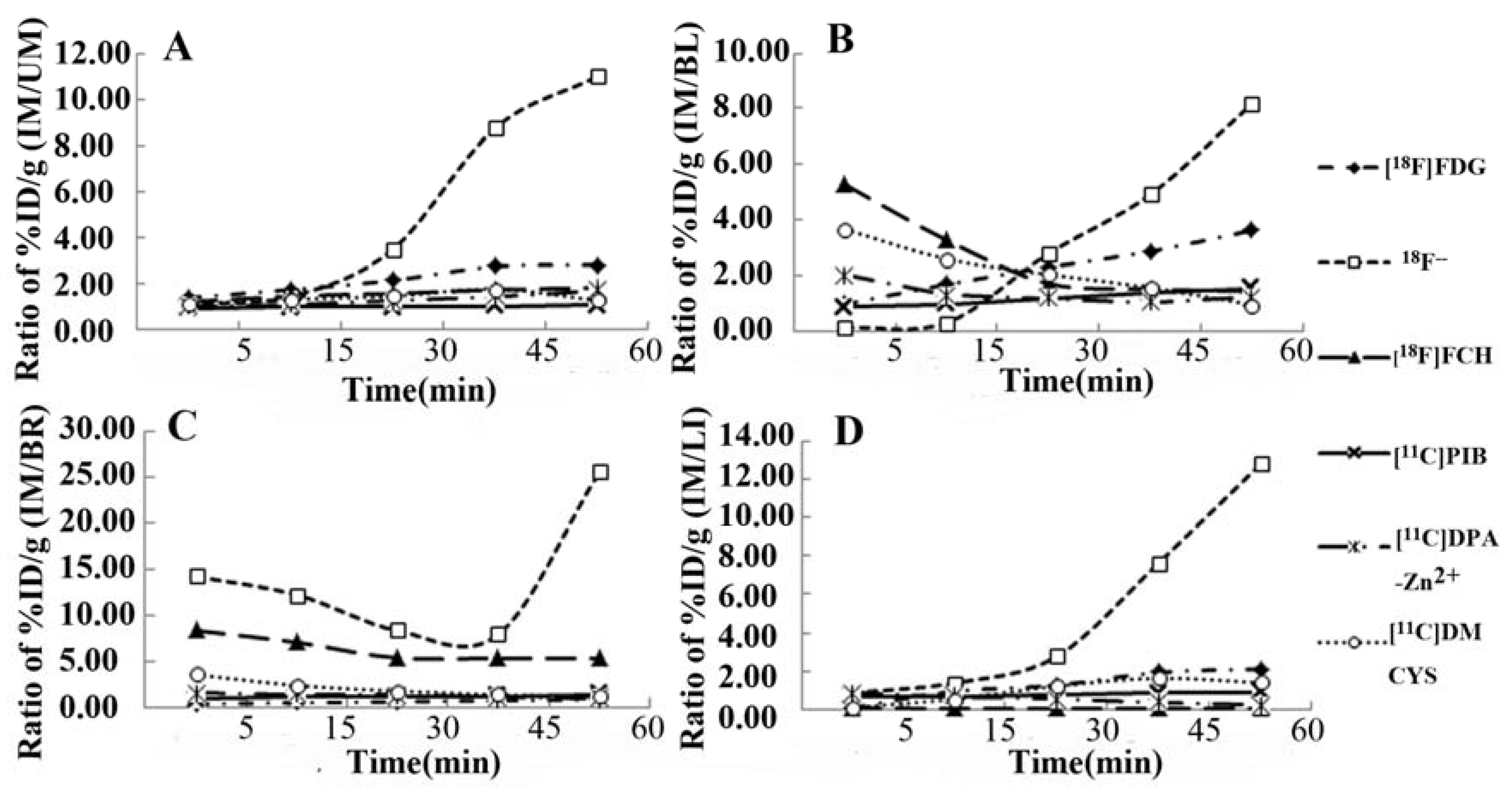
2.4. Tracer Uptake Ratio-Time Curves
2.5. Histopathologic Findings of Inflammation
3. Experimental
3.1. Generals
3.2. Radiosynthesis of PET Tracers
3.3. Inflammatory Mice Models
3.4. PET/CT Imaging
3.5. Time-Course of Tracer Uptake Ratios
3.6. Histopathological Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Conflict of Interest
References
- Phelps, M.E.; Huang, S.C.; Hoffman, E.J.; Selin, C.; Sokoloff, L.; Kuhl, D.E. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann. Neurol. 1979, 6, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.A.; Phelps, M.E.; Huang, S.C. Effects of temporal sampling, glucose metabolic rates, and disruptions of the blood-brain barrier on the FDG model with and without a vascular compartment: studies in human brain tumors with PET. Cereb. Blood Flow. Metab. 1986, 6, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Shreve, P.D.; Anzai, Y.; Wahl, R.L. Pitfalls in oncologic diagnosis with FDG PET imaging: physiologic and benign variants. Radiographics 1999, 19, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Strauss, L.G. Fluorine-18 deoxyglucose and false-positive results: A major problem in the diagnostics of oncological patients. Eur. J. Nucl. Med. 1996, 23, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Gotthardt, M.; Bleeker-Rovers, C.P.; Boerman, O.C.; Oyen, W.J.G. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J. Nucl. Med. 2010, 51, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Gambhir, S.S.; Czernin, J.; Schwimmer, J.; Silverman, D.H.; Coleman, R.E.; Phelps, M.E. A tabulated summary of the FDG PET literature. J. Nucl. Med. 2001, 42, 1S–93S. [Google Scholar] [PubMed]
- Liu, R.S.; Chou, T.K.; Chang, C.H.; Wu, C.Y.; Chang, C.W.; Chang, T.J.; Wang, S.J.; Lin, W.J.; Wang, H.E. Biodistribution, pharmacokinetics and PET Imaging of (18)F FMISO, (18)F FDG and (18)F FAc in a sarcoma- and inflammation-bearing mouse model. J. Nucl. Med. Biol. 2009, 36, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, M.; Nakamoto, Y.; Traughber, B.; Marshall, L.T.; Geschwind, J.F.H.; Wahl, R.L. Initial experience in small animal tumor Imaging with a clinical positron emission tomography/computed tomography scanner using 2- F-18 fluoro-2-deoxy-D-glucose. Cancer. Res. 2003, 63, 6252–6257. [Google Scholar] [PubMed]
- Vane, J.R.; Mitchell, J.A.; Appleton, I.; Tomlinson, A.; Bishop-Bailey, D.; Croxtall, J.; Willoughby, D.A. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc. Nad. Acad. Sci. USA 1994, 91, 2046–2050. [Google Scholar] [CrossRef]
- Kao, C.H.; Hsu, W.L.; Kao, P.F.; Lan, W.C.; Xie, H.L.; Lin, M.C.; Chao, H.Y. An efficient and aseptic preparation of “sodium fluoride (18F) injection” in a GMP compliant facility. Ann. Nucl. Med. 2010, 24, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Huang, T.T.; Liang, X.; Wu, K.N.; Tang, G.H. Semi-automatic Synthesis and PET imaging evaluation of [18F]fluoromethylcholine with the aseptic inflammation model. J. Isotopes 2011, 24, 198–204. [Google Scholar]
- Maeda, J.; Ji, B.; Irie, T.; Tomiyama, T.; Maruyama, M.; Okauchi, T.; Staufenbiel, M.; Iwata, N.; Ono, M.; Saido, T.C.; et al. Longitudinal, quantitative assessment of amyloid, neuroinflammation, and anti-amyloid treatment in a living mouse model of Alzheimer’s disease enabled by positron emission tomography. J. Neurosci. 2007, 27, 10957–10968. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Wang, H.L.; Jin, Y.F.; Liu, Y.H.; Tang, G.H.; Jiang, S.D. Synthesis and Radiolabelling of 18F FEDPA as an Imaging Agent for Apoptosis. Nucl. Radiochem. 2011, 33, 245–251. [Google Scholar]
- Yamada, S.; Kubota, K.; Kubota, R.; Ido, T.; Tamahashi, N. High accumulation of fluorine-18-fluorodeoxyglucose in turpentine-induced inflammatory tissue. J. Nucl. Med. 1995, 36, 1301–1306. [Google Scholar] [PubMed]
- Kubota, R.; Yamada, S.; Kubota, K.; Ishiwata, K.; Tamahashi, N.; Ido, T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J. Nucl. Med. 1992, 33, 1972–1980. [Google Scholar] [PubMed]
- Grant, F.D.; Fahey, F.H.; Packard, A.B.; Davis, R.T.; Alavi, A.; Treves, S.T. Skeletal PET with 18F-Fluoride: Applying New Technology to an Old Tracer. J. Nucl. Med. 2008, 49, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Choe, H.; Inaba, Y.; Kobayashi, N.; Ike, H.; Aoki, C.; Shizukuishi, K.; Iwamoto, N.; Yukizawa, Y.; Ishida, T.; Inoue, T.; et al. Use of F-18-fluoride PET to determine the appropriate tissue sampling region for improved sensitivity of tissue examinations in cases of suspected periprosthetic infection after total hip arthroplasty. Acta Orthop. 2011, 82, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Inaba, Y.; Choe, H.; Ike, H.; Fujimaki, H.; Tezuka, T.; Hirata, Y.; Tateishi, U.; Inoue, T.; Saito, T. Use of F-18 Fluoride PET to Differentiate Septic From Aseptic Loosening in Total Hip Arthroplasty Patients. Clin. Nucl. Med. 2011, 36, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, O.; Calabria, F.; Tavolozza, M.; Ciccio, C.; Carlani, M.; Caracciolo, C.R.; Danieli, R.; Orlacchio, A.; Simonetti, G. F-18-choline PET/CT physiological distribution and pitfalls in image interpretation: experience in 80 patients with prostate cancer. Nucl. Med. Commun. 2010, 31, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Furumoto, S.; Iwata, R.; Fukuda, H.; Kawamura, K.; Ishiwata, K. Comparison of F-18-fluoromethylcholine and F-18-2-deoxy-D-glucose in the distribution of tumor and inflammation. Ann. Nucl. Med. 2006, 20, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Talbot, J.N.; Gutman, F.; Fartoux, L.; Grange, J.D.; Ganne, N.; Kerrou, K.; Grahek, D.; Montravers, F.; Poupon, R.; Rosmorduc, O. PET/CT in patients with hepatocellular carcinoma using [18F]fluorocholine: preliminary comparison with [18F]FDG PET/CT. Eur. J. Nucl. Med. 2006, 33, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Wyss, M.T.; Weber, B.; Honer, M.; Spath, N.; Ametamey, S.M.; Westera, G.; Bode, B.; Kaim, A.H.; Buck, A. F-18-choline in experimental soft tissue infection assessed with autoradiography and high-resolution PET. Eur. J. Nucl. Med. 2004, 31, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.F.; Tang, X.L.; Wang, H.L.; Tang, G.H.; Wen, F.H.; Shi, X.C.; Yi, C.; Wu, K.N.; Meng, Q.F. S-C-11-Methyl-L-Cysteine: A New Amino Acid PET Tracer for Cancer Imaging. J. Nucl. Med. 2011, 52, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.; Wang, H.L.; Tang, G.H. Progress of small-molecule PET probes for apoptosis imaging. J. Isotopes 2011, 24, 181–185. [Google Scholar]
- Thakur, M.L.; Zhang, K.; Paudyal, B.; Devakumar, D.; Covarrubias, M.Y.; Cheng, C.; Gray, B.D.; Wickstrom, E.; Pak, K.Y. Targeting Apoptosis for Optical Imaging of Infection. Mol. Imaging Biol. 2012, 14, 163–171. [Google Scholar] [CrossRef] [PubMed]
- White, A.G.; Fu, N.; Leevy, W.M.; Lee, J.J.; Blasco, M.A.; Smith, B.D. Optical Imaging of Bacterial Infection in Living Mice Using Deep-Red Fluorescent Squaraine Rotaxane Probes. Bioconjug. Chem. 2010, 21, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.H.; Tang, X.L.; Deng, H.F.; Wang, H.L.; Wen, F.H.; Yi, C.; Wu, K.N. Efficient preparation of [11C]CH3Br for the labeling of [11C]CH3-containing tracers in positron emission tomography clinical practice. Nucl. Med. Commun. 2011, 32, 466–474. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


| Tissue | [18F]FDG | 18F− | [18F]FCH | [11C]PIB | [11C]DPA-Zn2+ | [11C]DMCYS |
|---|---|---|---|---|---|---|
| Blood | 1.36 ± 0.14 | 0.54 ± 0.12 | 1.17 ± 0.08 | 0.47 ± 0.08 | 1.36 ± 0.12 | 1.58 ± 0.52 |
| Brain | 4.99 ± 0.39 | 0.17 ± 0.02 | 0.30 ± 0.01 | 0.23 ± 0.02 | 1.27 ± 0.35 | 1.45 ± 0.48 |
| Heart | 12.5 ± 2.23 | 0.52 ± 0.03 | 2.50 ± 0.35 | 0.40 ± 0.11 | 0.61 ± 0.07 | 1.16 ± 0.68 |
| Lung | 2.2 ± 0.68 | 0.41 ± 0.05 | 3.11 ± 0.47 | 0.31 ± 0.05 | 0.66 ± 0.18 | 1.35 ± 0.73 |
| Liver | 1.88 ± 0.27 | 0.35 ± 0.02 | 20.51 ± 12.24 | 0.33 ± 0.04 | 3.31 ± 1.09 | 1.10 ± 0.28 |
| Stomach | 1.51 ± 0.31 | 0.28 ± 0.02 | 2.30 ± 0.31 | 0.30 ± 0.06 | 0.60 ± 0.14 | 0.91 ± 0.42 |
| Pancreas | 3.11 ± 0.38 | 0.41 ± 0.01 | 4.26 ± 0.67 | 0.39 ± 0.11 | 0.41 ± 0.06 | 4.17 ± 1.54 |
| Kidney | 3.89 ± 0.16 | 0.61 ± 0.05 | 8.00 ± 1.69 | 0.29 ± 0.05 | 1.29 ± 0.39 | 0.79 ± 0.35 |
| Intestine | 2.22 ± 0.34 | 0.39 ± 0.08 | 5.09 ± 0.72 | 0.33 ± 0.07 | 2.73 ± 0.85 | 1.08 ± 0.29 |
| Femur | 3.78 ± 2.53 | 21.97 ± 8.02 | 2.19 ± 0.25 | 0.06 ± 0.02 | 0.19 ± 0.03 | 0.12 ± 0.03 |
| Muscle | 1.48 ± 1.22 | 0.42 ± 0.04 | 0.97 ± 0.01 | 0.31 ± 0.04 | 0.83 ± 0.13 | 1.24 ± 0.46 |
| Inflammation | 4.33 ± 1.44 | 4.72 ± 0.70 | 1.78 ± 0.02 | 0.31 ± 0.03 | 1.69 ± 0.15 | 1.64 ± 0.11 |
| IM/BL a | 3.18 ± 0.28 | 8.74 ± 1.58 | 1.52 ± 0.26 | 0.64 ± 0.13 | 1.24 ± 0.27 | 0.99 ± 0.18 |
| IM/NM b | 2.93 ± 0.31 | 11.23 ± 2.32 | 1.83 ± 0.51 | 1.01 ± 0.09 | 1.73 ± 0.32 | 1.32 ± 0.25 |
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, T.; Wang, H.; Tang, G.; Liang, X.; Nie, D.; Yi, C.; Wu, K. A Comparative Uptake Study of Multiplexed PET Tracers in Mice with Turpentine-Induced Inflammation. Molecules 2012, 17, 13948-13959. https://doi.org/10.3390/molecules171213948
Huang T, Wang H, Tang G, Liang X, Nie D, Yi C, Wu K. A Comparative Uptake Study of Multiplexed PET Tracers in Mice with Turpentine-Induced Inflammation. Molecules. 2012; 17(12):13948-13959. https://doi.org/10.3390/molecules171213948
Chicago/Turabian StyleHuang, Tingting, Hongliang Wang, Ganghua Tang, Xiang Liang, Dahong Nie, Chang Yi, and Kening Wu. 2012. "A Comparative Uptake Study of Multiplexed PET Tracers in Mice with Turpentine-Induced Inflammation" Molecules 17, no. 12: 13948-13959. https://doi.org/10.3390/molecules171213948




