An Improved Method for Removal of Azo Dye Orange II from Textile Effluent Using Albumin as Sorbent
Abstract
:1. Introduction
2. Results and Discussion
2.1. Influence of the Time Interaction, pH and Adsorption
| Assay | Azo dye/albumin concentration (g/L) | Time of incubation (min.) | pH | Orange II removal (%) |
|---|---|---|---|---|
| 01 | 0.10 | 20 | 3.0 | 10.72 |
| 02 | 0.10 | 30 | 3.0 | 21.49 |
| 03 | 0.10 | 60. | 3.0 | 54.06 |
| 04 | 0.10 | 90. | 3.0 | 87.28 |
| 05 | 0.10 | 60 | 3.2 | 78.95 |
| 06 | 0.10 | 60 | 3.5 | 84.20 |
| 07 | 0.10 | 60 | 3.8 | 63.40 |
| 08 | 0.10 | 60 | 4.0 | 55.71 |
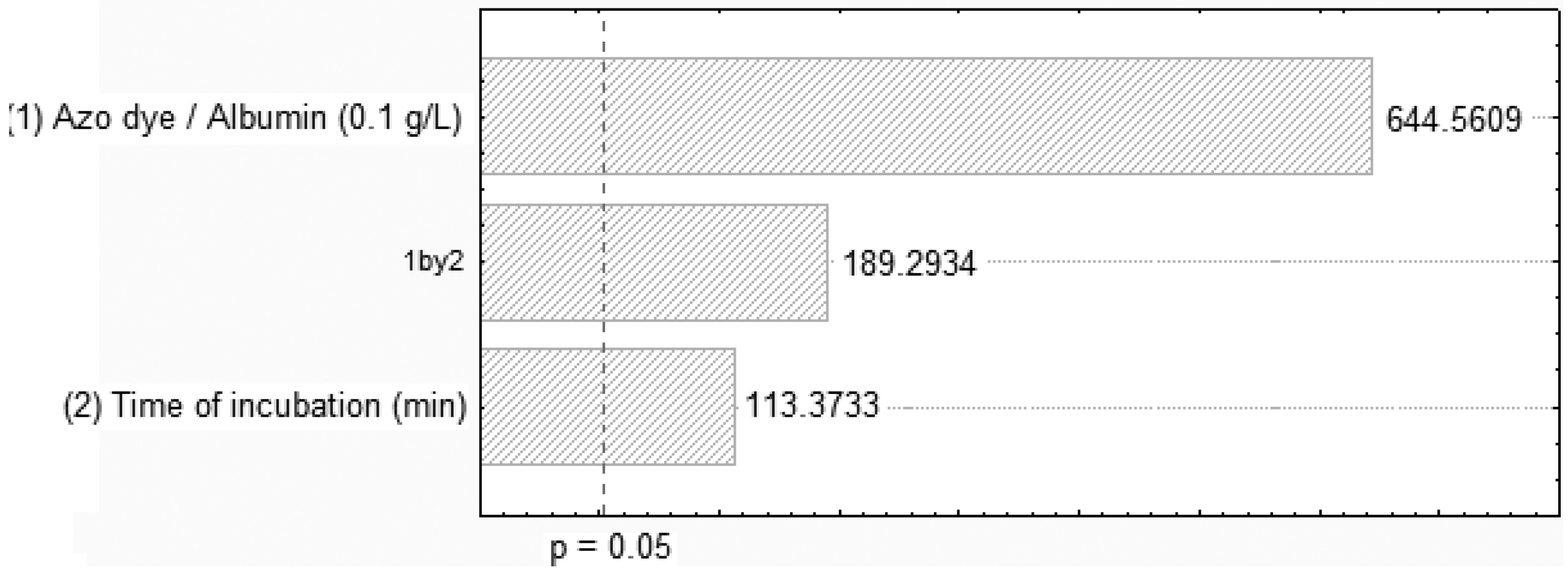
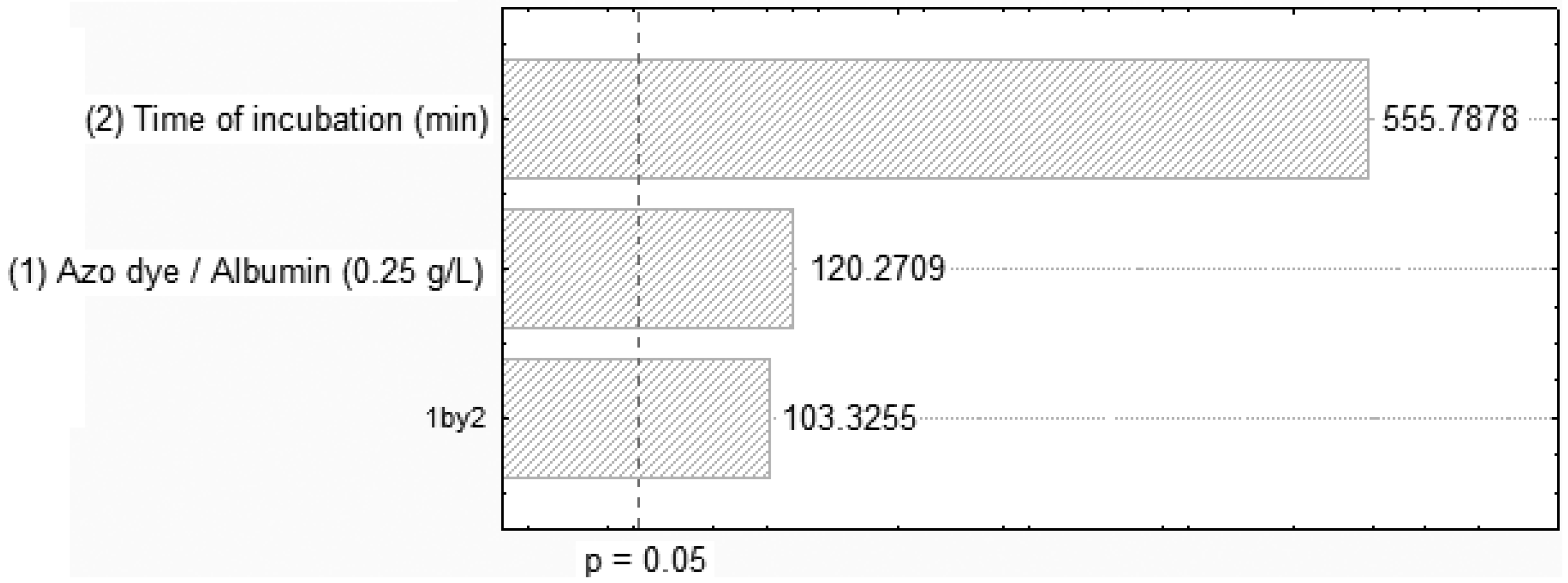
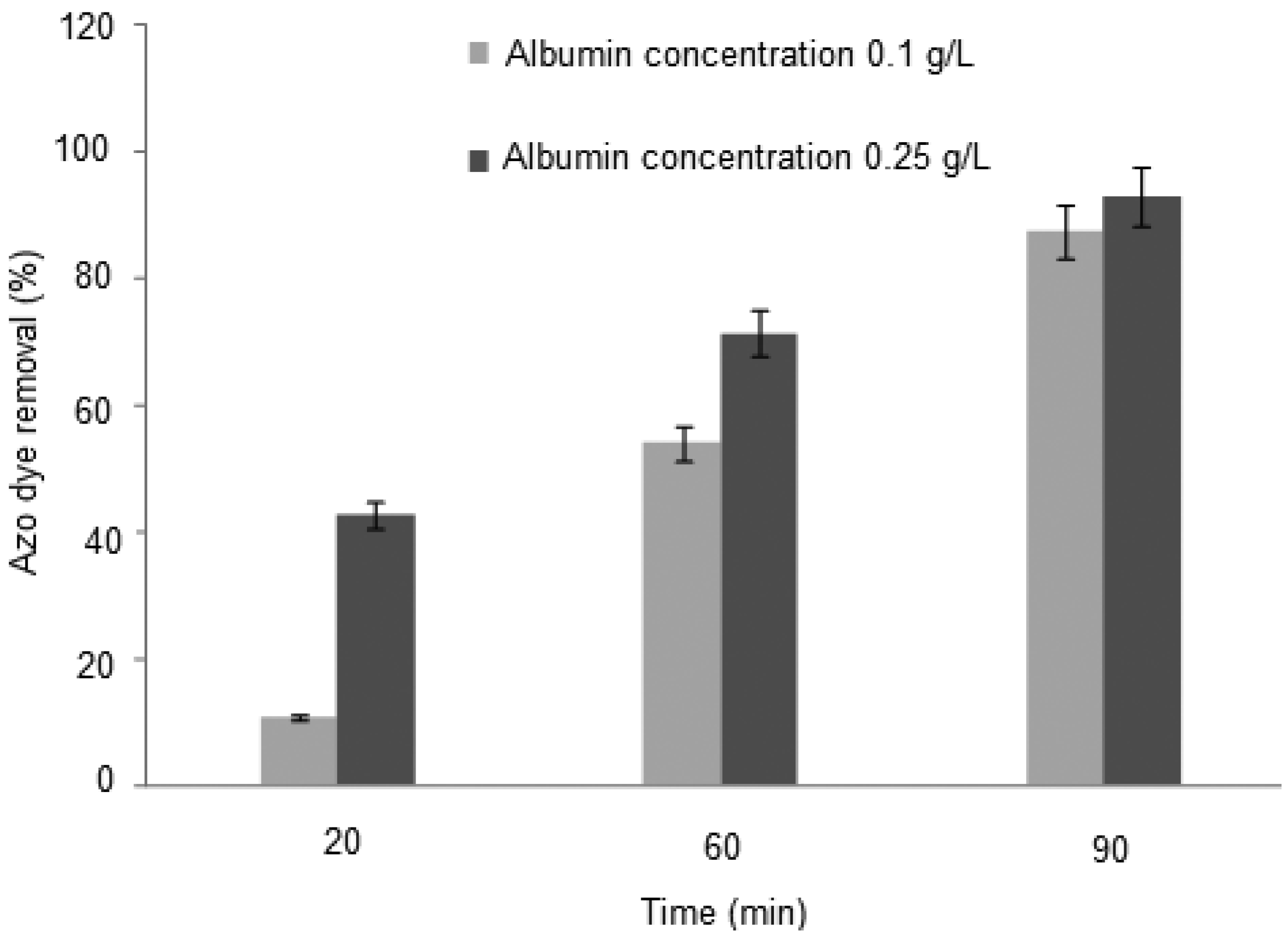
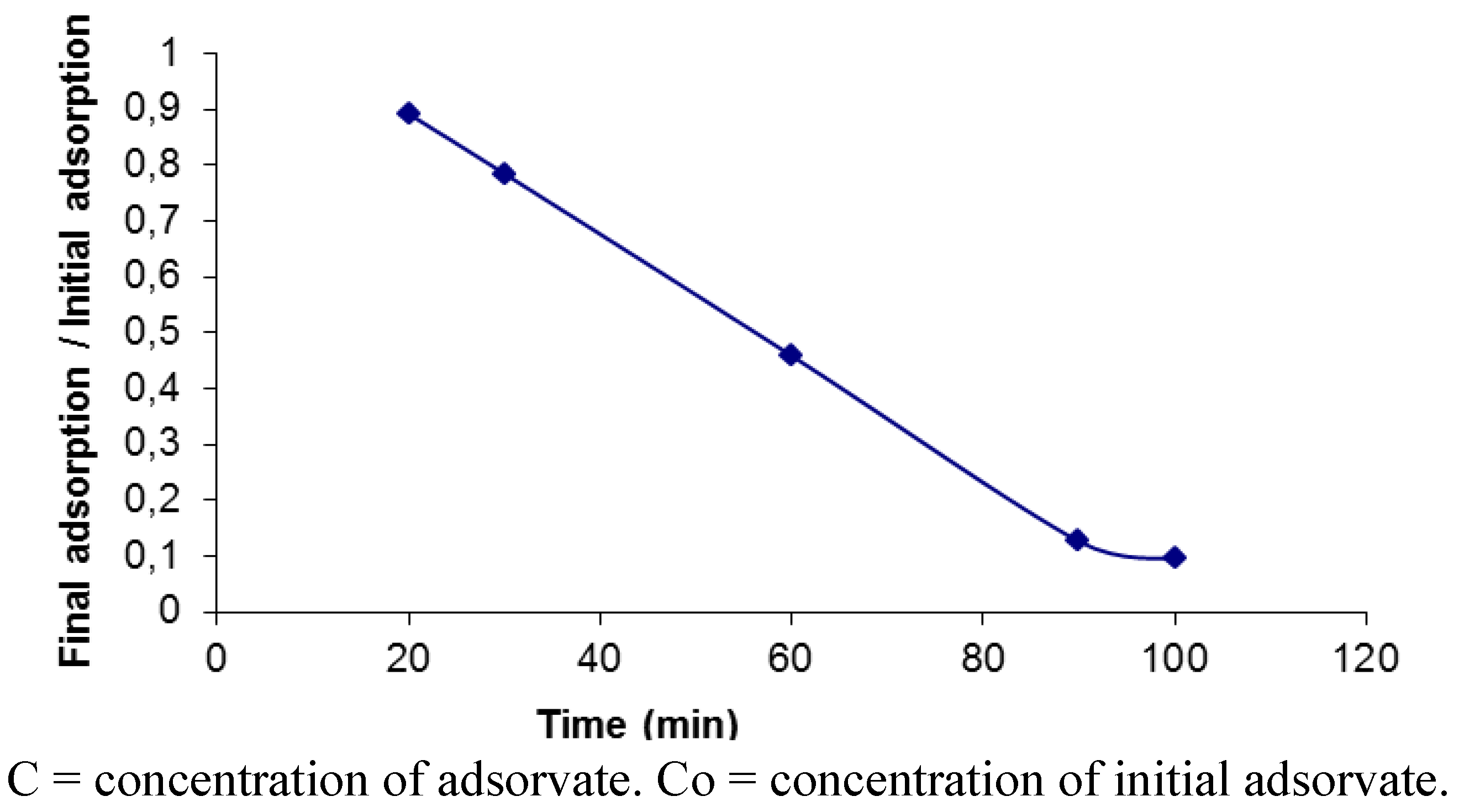
2.2. Removal of Orange II in the Azo Dye/Albumin (v/v) System, and Time of Interaction
| Assay | Azo dye/albumin proportions (mL) | Orange II removal (%) |
|---|---|---|
| 01 | 1:1 (25 + 25) | 27.48 |
| 02 | 1:2 (25 + 50) | 56.21 |
| 03 | 1:3 (25 + 75) | 84.20 |
| 04 | 1:4 (25 + 100) | 33.33 |
| 05 | 1:5 (25 + 125) | 27.85 |
| Assay | Relation dye/albumin v/v (mL) | Contact time (min) | pH before filtration | pH after filtration | Retention time % |
|---|---|---|---|---|---|
| 1 | 50/50 | 60 | 3.5 | 3.2 | 27.84 |
| 2 | 50/100 | 60 | 3.5 | 3.2 | 33.83 |
| 3 | 50/150 | 60 | 3.5 | 3.2 | 85.00 |
| 4 | 50/200 | 60 | 3.5 | 3.2 | 69.57 |
| 5 | 50/250 | 60 | 3.5 | 3.2 | 77.28 |
| 6 | 25/75 | 20 | 3.5 | 3.9 | 46.81 |
| 7 | 25/100 | 20 | 3.5 | 3.6 | 66.67 |
| 8 | 25/125 | 20 | 3.5 | 3.7 | 13.34 |
| 9 | 25/75 | 20 | 3.0 | 3.0 | 46.16 |
| 10 | 25/75 | 20 | 3.1 | 3.1 | 21.96 |
| 11 | 25/75 | 20 | 3.2 | 3.2 | 21.43 |
| 12 | 25/75 | 20 | 3.3 | 3.3 | 24.45 |
| 13 | 25/75 | 80 | 3.6 | 3.6 | 54.77 |
| 14 | 8.5/34 | 80 | 3.5 | 3.5 | 90.33 |
| 15 | 12/48 | 60 | 3.5 | 3.5 | 97.15 |
| 16 | 25/75 | 60 | 3.5 | 3.5 | 99.50 |
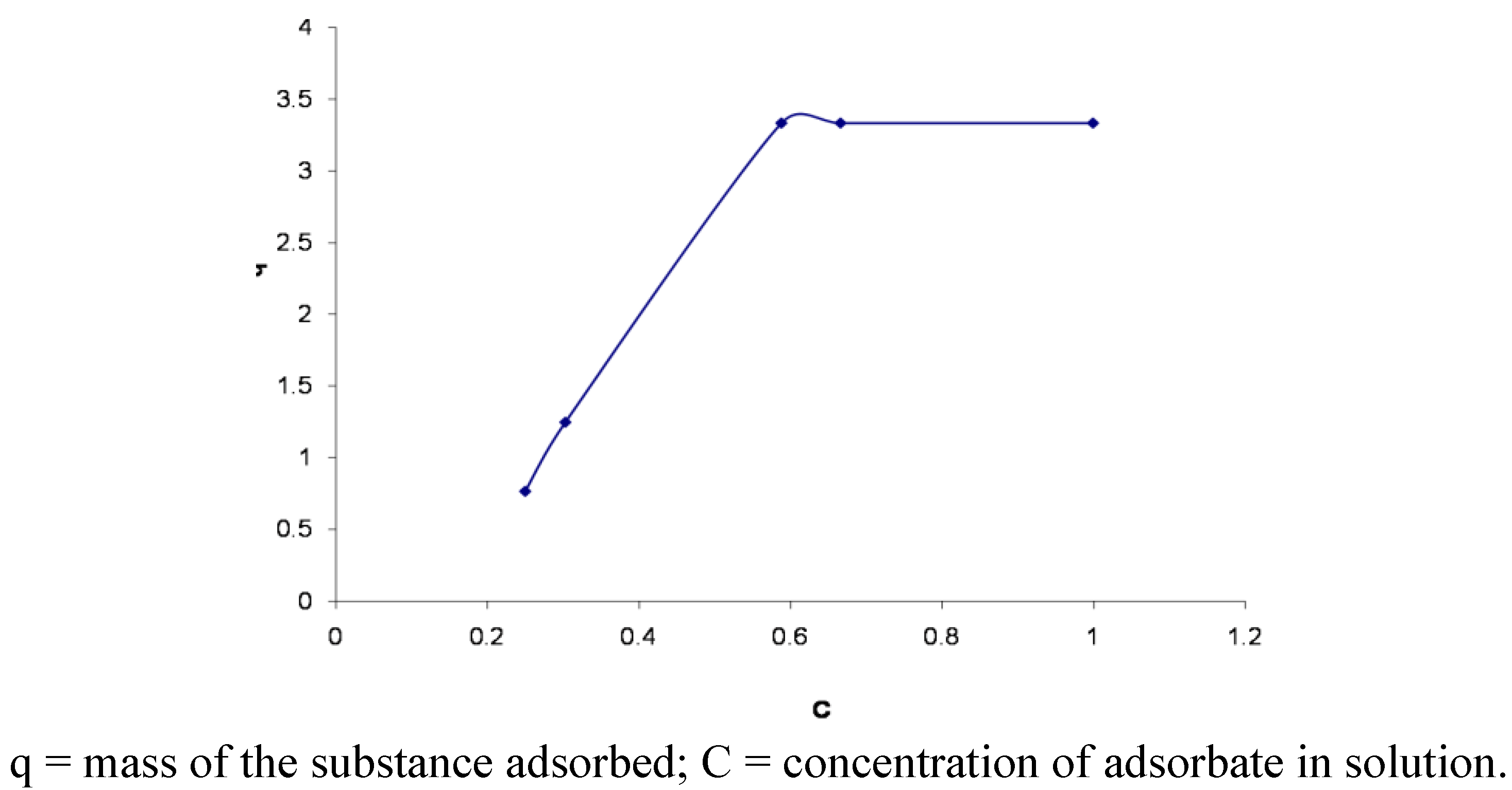

3. Experimental
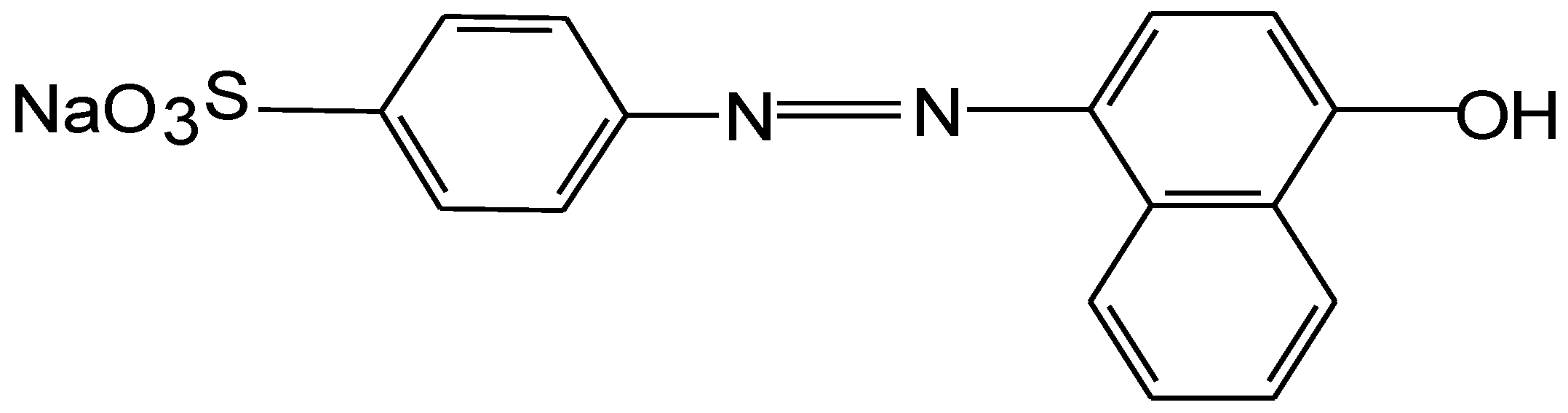
3.1. Dye, Effluent, Albumin and Membrane
3.2. Vacuum Filtration Procedure
3.3. Colour Reduction Measurement
3.4. Albumin Determination
3.5. Experimental Factorial Design
3.6. Adsorption Isotherms
| First full factorial design | |||
| Factor | Level | ||
| −1 | 0 | +1 | |
| Azo dye/Albumin0.10 g/L (v/v) | 1:3 | 1:2 | 1:1 |
| Time of incubation (min) | 30 | 60 | 90 |
| Second full factorial design | |||
| Factor | Level | ||
| −1 | 0 | +1 | |
| Azo dye/Albumin0.25 g/L (v/v) | 1:3 | 1:2 | 1:1 |
| Time of incubation (min) | 30 | 60 | 90 |
4. Conclusions
Acknowledgments
References
- Ambrosio, S.T.; Campos-Takaki, G.M. Decolorization of reactive azo dyes by Cunninghamella elegans UCP 542 under co-metabolism conditions. Bioresour. Technol. 2004, 91, 69–75. [Google Scholar] [CrossRef]
- Zollinger, H. Color Chemistry, 2nd ed; VCH Publishers: New York, NY, USA, 1991. [Google Scholar]
- Guaratini, C.I.; Zanoni, M.V. Textile Dyes. Quím. Nova 2000, 23, 71–78. [Google Scholar] [CrossRef]
- Zamora, P.; Kunz, A.P.; Moraes, S.G.; Duran, N. New tendencies on textile effluent treatment. Quím. Nova 2002, 25, 78–82. [Google Scholar] [CrossRef]
- Zanoni, M.V.B.; Sousa, W.R.; Lima, J.P.; Carneiro, P.A.; Fogg, A.G. Application of voltammetric technique to the analysis of indanthrene dye in alckaline solution. Dyes Pigm. 2006, 68, 19–25. [Google Scholar] [CrossRef]
- Allinger, N.L.; Cava, M.P.; de Jongh, D.C.; Johnson, C.R.; Lebel, N.A.; Stevens, C.L. Organic Chemistry; Worth Publishers: New York, NY, USA, 1976. [Google Scholar]
- Araújo, M.; Castro, E.M.M. Manual de Engenharia Têxtil; Fundação Calouste Gulbenkian: Lisbon, Portugal, 1984. [Google Scholar]
- Daltin, D.; Alcantra, M.R. Textile Chemistry. Quim. Nova 1996, 19, 320–330. [Google Scholar]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Erdal, S.; Taskin, M. Uptake of textile dye Reactive Black-5 by PenicilliumchrysogenumMT-6 isolated from cement-contaminated soil. Afr. J. Microbiol. Res. 2010, 4, 618–625. [Google Scholar]
- Anliker, R. Organic Colorants—Interpretation of Mammalian, Genus and Ecotoxicity Data in Terms of Potential Risks; The Royal Society of Chemistry: London, UK, 1986. [Google Scholar]
- Correia, V.M.; Stephenson, T.; Judd, S.J. Characterization of textile wastewaters. Environ. Technol. 1994, 15, 917–929. [Google Scholar] [CrossRef]
- Bielska, M.; Prochasca, K.J. Dyes separation by means of cross-flow ultrafiltration of micellar solution. Dyes Pigm. 2007, 74, 410–415. [Google Scholar] [CrossRef]
- Anjaneyulu, Y.; Chary, N.; Raj, D.; Samuel, S. Decolorization of industrial effluents—Available methods and emerging Technologies—A review. Rev. Environ. Sci. Biotechnol. 2005, 4, 245–273. [Google Scholar]
- Beck, J.V. Nonlinear estimation applied to the nonlinear heat conduction. J. Heat Transfer 1988, 13, 1046–1058. [Google Scholar] [CrossRef]
- Al-Malack, M.H.; Anderson, G.K. Coagulation—Cross flow micro filtration of domestic waste water. J. Memb. Sci. 1996, 121, 59–65. [Google Scholar]
- Dogan, M.; Alkan, M. Adsorption kinetics of methyl violet onto perlite. Chemosphere 2003, 50, 517–528. [Google Scholar] [CrossRef]
- Haan, J. The renal function as judged by the excretion of vital dye-stuffs. J. Physiol. 1922, 56, 444–450. [Google Scholar]
- Pond, S.M. Extracorporeal techniques in the treatment of poisoned patients. Med. J. Aust. 1991, 154, 617–622. [Google Scholar]
- Holm, N. Aggregation and fibrillation of bovine serum albumin. Biochim. Biophys. Acta 2007, 1774, 1128–1138. [Google Scholar]
- Vetri, V.; Librizzi, L.; Leone, L.; Militello, V. Thermal aggregation of bovine serum albumin at different pH: Comparison with human serum albumin. Eur. Biophys. J. 2007, 36, 717–725. [Google Scholar] [CrossRef]
- Gao, D.; Tian, Y.; Liang, F.; Jim, D.; Chen, Y.; Zhang, H.; Yu, A. Investigation on the pH-dependent binding of EosinY and bovine serum albumin by spectral methods. J. Lumin. 2007, 127, 515–522. [Google Scholar] [CrossRef]
- Jayabharathi, J.; Thamikachalam, M.P. Mechanistic investigation on binding of bioactive imidazole with protein bovine serum albumin—A biophysical study. Spectrochim. Acta A 2011, 79, 502–507. [Google Scholar] [CrossRef]
- Doyen, W. Latest developments in ultrafiltration for large-scale drinking water applications. Desalination 1997, 113, 165–177. [Google Scholar]
- Hamza, A.; Pham, V.A.; Matsuura, T.; Santerre, J.P. Development of membranes with low surface energy to reduce the fouling in filtration application. J. Memb. Sci 1997, 131, 217–227. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Sharma, A. Adsorption characteristics of the dye, Brilliant Green, on Neem leaf powder. Dyes Pigm. 2003, 57, 211–222. [Google Scholar] [CrossRef]
- Bader, M.S.H.; Veenstra, J.N. Analysis of concentration polarization phenomenon in ultrafiltration under turbulent flux conditions. J. Memb. Sci. 1996, 114, 139–148. [Google Scholar] [CrossRef]
- Darup, J.; Bleese, N.; Kalmar, P.; Lutz, G.; Pokar, H. Hemofiltration during extracorporeal circulation (ECC). J. Thorac. Cardiovasc. Surg. 1979, 27, 227–330. [Google Scholar] [CrossRef]
- Laurence, J.D.R. A study of the adsorption of dyes on bovine serum albumin by the method of polarization of fluorescence. Biochem. J. 1952, 51, 168–180. [Google Scholar]
- Nicolaisen, B. Developments in membrane technology for water treatment. Desalination 2002, 153, 355–360. [Google Scholar] [CrossRef]
- Shu, H.Y.; Shang, M. Pilot scale annular plug flow photoreactor by UV/H2O2 for the decolorization of azo dye wastewater. J. Hazard. Mater. 2005, 125, 244–251. [Google Scholar] [CrossRef]
- Shu, H.Y.; Shang, M.C. Decolorization and mineralization of a phthalocyanine dye C.I. Direct Blue 199 using UV/H2O2 process. J. Hazard. Mater. 2005, 125, 96–101. [Google Scholar] [CrossRef]
- Mu, Y.; Rabaey, K.; Rozendal, R.A.; Yuan, Z.; Keller, J. Decolorization of azo dyes in bioelectrochemical systems. Environ. Sci. Technol. 2009, 43, 5137–5143. [Google Scholar] [CrossRef]
- Sereikaite, J.; Bumelis, V. Interaction of serum albumin with vinyl sulfonate azo dye. Cent. Eur. J. Chem. 2008, 6, 509–512. [Google Scholar] [CrossRef]
- Woei-Jye, L.; Ismail, A.F. Polymeric nanofiltration membranes for textile dye wastewater treatment: Preparation, performance evaluation, transport modelling, and fouling control—A review. Desalination 2009, 245, 321–348. [Google Scholar] [CrossRef]
- Namasivayan, C.; Kavitha, D. Removal of Congo Red from water by adsorption onto activated carbon prepared from coir pith, an agricultural solid waste. Dyes Pigm. 2002, 54, 47–58. [Google Scholar] [CrossRef]
- Robinson, T.; Chandran, B.; Nigam, P. Removal of dyes from an artificial textile dye effluent by two agricultural waste residues. Environ. Int. 2002, 28, 29–33. [Google Scholar] [CrossRef]
- Zhang, M.M.; Chang, C.T.; Ding, Y.; Lin, K.L.; Chiou, C.S.; Hsueh, C.C.; Xu, H. Assessment upon azo dye decolorization and bioelectricity generation by Proteus hauseri. Bioresour. Technol. 2010, 101, 4737–4741. [Google Scholar]
- Zhao, J.; Chen, J.; Gao, J. Decolorization of Azo Dyes C.I. Acid Yellow 17 and C.I.Direct Red 31 by Dielectric-Barrier Discharge Air Plasma. Plasma Sci. 2010, 8, 488–495. [Google Scholar]
- Mclaughlin, J.L.; Saizarbitori, T.-C.; Anderson, J.E. Trés bioensayos simples para quimicos de productos naturales. Rev. Soc. Venez. Quim. 1995, 18, 13–18. [Google Scholar]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2005, 97, 1061–1085. [Google Scholar] [CrossRef]
- Kumar, K.V.; Sivanesan, S. Equilibrium data, isotherm parameters and process design for partial and complete isotherm of methylene blue onto activated carbon. J. Hazard. Mater. 2006, 134, 237–244. [Google Scholar] [CrossRef]
- StatSoft, Inc. Statistica, version 7.1; Data analysis software system. Tulsa, OK, USA, 2005.
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ohashi, T.; Jara, A.M.T.; Batista, A.C.L.; Franco, L.O.; Lima, M.A.B.; Benachour, M.; Da Silva, C.A.A.; Campos-Takaki, G.M. An Improved Method for Removal of Azo Dye Orange II from Textile Effluent Using Albumin as Sorbent. Molecules 2012, 17, 14219-14229. https://doi.org/10.3390/molecules171214219
Ohashi T, Jara AMT, Batista ACL, Franco LO, Lima MAB, Benachour M, Da Silva CAA, Campos-Takaki GM. An Improved Method for Removal of Azo Dye Orange II from Textile Effluent Using Albumin as Sorbent. Molecules. 2012; 17(12):14219-14229. https://doi.org/10.3390/molecules171214219
Chicago/Turabian StyleOhashi, Tadashi, Alícia M. T. Jara, Anabelle C. L. Batista, Luciana O. Franco, Marcos A. Barbosa Lima, Mohand Benachour, Carlos A. Alves Da Silva, and Galba M. Campos-Takaki. 2012. "An Improved Method for Removal of Azo Dye Orange II from Textile Effluent Using Albumin as Sorbent" Molecules 17, no. 12: 14219-14229. https://doi.org/10.3390/molecules171214219




