Toxicological Assessment of ?-(1à6)-Glucan (Lasiodiplodan) in Mice during a 28-Day Feeding Study by Gavage
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biochemical Alterations Caused by Lasiodiplodan Intake
2.2. Lipid Profile Arising from Administering Lasiodiplodan to Mice
2.3. Hematological Parameters
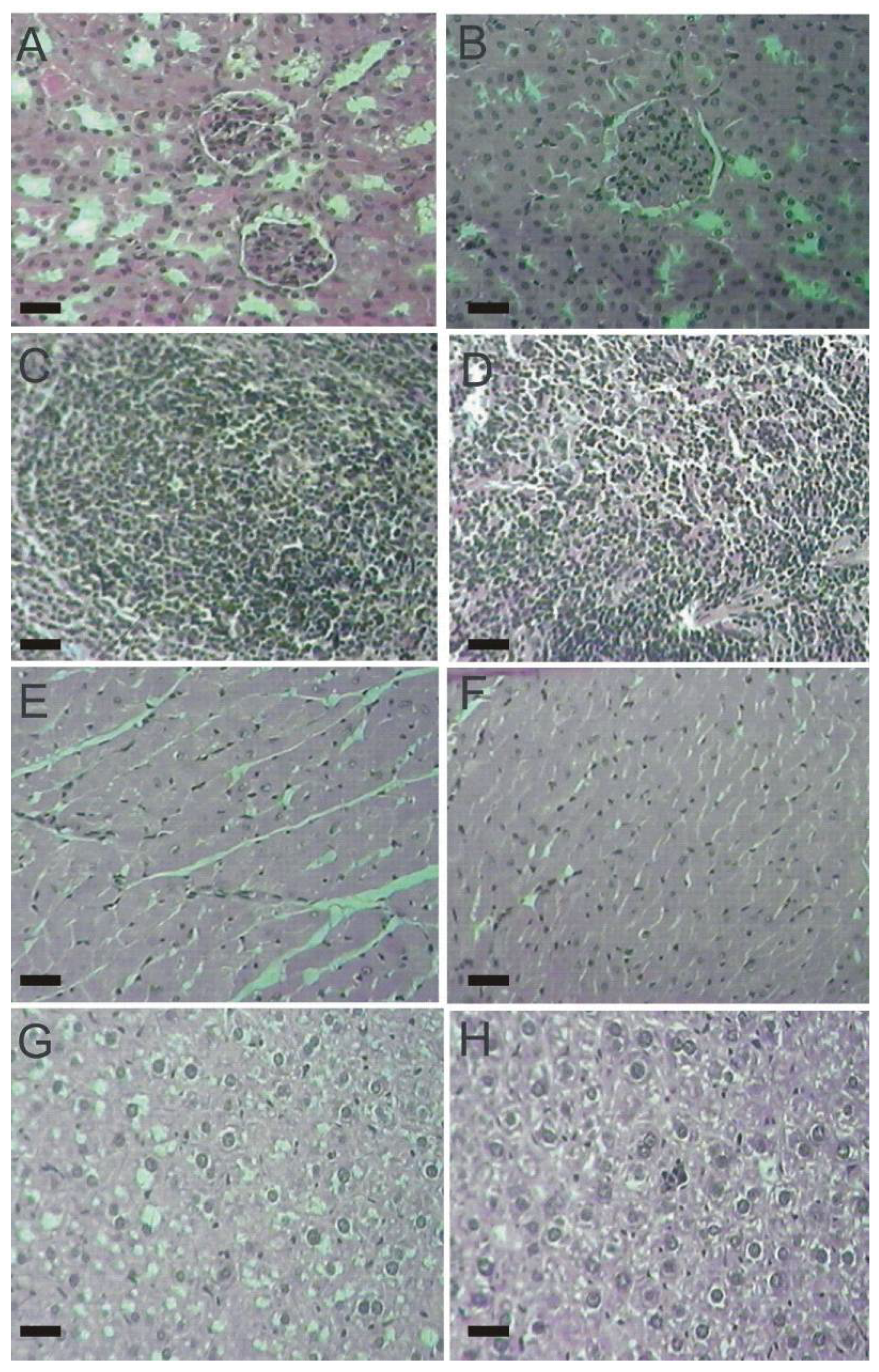
2.4. Histopathological Parameters
3. Experimental
3.1. Microorganism and Cultivation
3.2. Recovery of Exopolysaccharide
3.3. Preparation of the Lasiodiplodan Stock Solution
3.4. Experimental Design
3.5. Biochemical and Hematological Analyses
3.6. Histopathological Analysis
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Novak, M.; Vetvicka, V. β-Glucans, history, and the present: Immunomodulatory aspects and mechanism of action. J. Immunotoxicol. 2008, 5, 47–57. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, D.; Cuda, C.; Luhovyy, B.L.; Anderson, G.H. Beta glucan: Health benefits in obesity and metabolic syndrome. J. Nutr. Metabol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, L.; Phillips, F.; O’Sullivan, K.; Walton, J. Wheat bran: Its composition and benefits to health, a European perspective. Int. J. Food Sci. Nutr. 2012, 63, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Raymond, K. Beta-glucans in the treatment of diabetes and associated cardiovascular risks. Vasc. Health Risk Manag. 2008, 4, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Seviour, R.J. Medicinal importance of fungal β-(1→3);(1→6)-glucans. Mycol. Res. 2007, 111, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Serviour, R.J.; McNeil, B.; Fazenda, M.L.; Harvey, L.M. Operating bioreactors for microbial exopolysaccharide production. Crit. Rev. Biotechnol. 2011, 31, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Bohn, J.A.; BeMiller, J.N. (1→3)-β-d-Glucans as biological response modifiers: A review of structure-functional activity relationships. Carbohydr. Polym. 1995, 28, 3–14. [Google Scholar] [CrossRef]
- Soltanian, S.; Stuyven, E.; Cox, E.; Sorgeloos, P.; Bossier, P. Beta-glucans as immunostimulant in vertebrates and invertebrates. Crit. Rev. Microbiol. 2009, 35, 109–138. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.C.B.O.; Dekker, R.F.H.; Serpeloni, J.M.; Fonseca, E.A.I.; Colus, I.S.; Barbosa, A.M. Anticlastogenic activity exhibited by botryosphaeran, a new exopolysaccharide produced by Botryosphaeria rhodina MAMB-05. Int. J. Biol. Macromol. 2008, 42, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Kiho, T.; Morimoto, H.; Sakushima, M.; Usui, S.; Ukai, S. Polysaccharides in fungi. XXXV. Anti diabetic activity of an acidic polysaccharide from the fruiting bodies of Tremella aurantia. Biol. Pharm. Bull. 1995, 18, 1627–1629. [Google Scholar] [CrossRef] [PubMed]
- Nicolosi, R.; Bell, S.J.; Bistrian, B.R.; Greenberg, I.; Forse, R.A.; Blackburn, G.L. Plasma lipid changes after supplementation with beta-glucan fiber from yeast. Am. J. Clin. Nutr. 1999, 70, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Kim, S.W.; Lim, J.M.; Joo, J.H.; Kim, H.O.; Kim, H.M.; Yun, J.W. Hypoglycemic effect of crude exopolysaccharides produced by a medicinal mushroom Phellinus baumii in streptozotocin-induced diabetic rats. Life Sci. 2005, 76, 3069–3080. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Nantes, C.C.B.O.; Fonseca, E.A.I.; Zaia, C.T.B.V.; Dekker, R.F.H.; Khaper, N.; Castro, I.A.; Barbosa, A.M. Hypoglycemic and hypocholesterolemic effects of botryosphaeran from Botryosphaeria rhodina MAMB-05 in diabetes-induced and hyperlipidemia conditions in rats. Mycobiology 2011, 39, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Narui, T.; Sawada, K.; Culberson, C.F.; Culberson, W.L.; Shibata, S. Pustulan-type polysaccharides as a constant character of the umbilicariaceae (Lichenized Ascomycotina). Bryologist 1999, 102, 80–85. [Google Scholar] [CrossRef]
- Sassaki, G.L.; Ferreira, J.C.; Glienke-Branco, C.; Torri, G.; Toni, F.D.; Gorin, P.A.J.; Iacomini, M. Pustulan and branched β-galactofuranan from the phytopathogenic fungus Guignardia citricarpa, excreted from media containing glucose and sucrose. Carbohydr. Polym. 2002, 48, 385–389. [Google Scholar] [CrossRef]
- Vasconcelos, A.F.; Monteiro, N.; Dekker, R.F.H.; Barbosa, A.M.; Carbonero, E.; Silveira, J.L.; Sassaki, G.L.; Silva, R.; Conradi da Silva, M.L. Three exopolysaccharides of the β-(1→6)-d-glucan type and a β-(1→3;1→6)-d-glucan produced by strains of Botryosphaeria rhodina isolated from rotting tropical fruit. Carbohydr. Res. 2008, 343, 2481–2485. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.A.A.; Túrmina, J.A.; Ivanov, R.C.; Barroso, R.R.; Marques, P.T.; Fonseca, E.A.I.; Fortes, Z.B.; Dekker, R.F.H.; Khaper, N.; Barbosa, A.M. Lasiodiplodan, an exocellular β-(1→6)-d-glucan from Lasiodiplodia theobromae MMPI: Production on glucose, fermentation kinetics, rheology and anti-proliferative activity. J. Ind. Microbiol. Biotechnol. 2012, 39, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Delaney, B.; Carlson, T.; Frazer, S.; Zheng, T.; Hess, R.; Ostergren, K.; Kierzek, K.; Haworth, J.; Knutson, N.; Junker, K.; et al. Evaluation of the toxicity of concentrated barley β-glucan in a 28-day feeding study in Wistar rats. Food Chem. Toxicol. 2003, 41, 477–487. [Google Scholar] [CrossRef]
- Kiho, T.; Hui, J.; Yamane, A.; Ukai, S. Polysaccharides in fungi. XXXII. Hypoglycemic activity and chemical properties of a polysaccharide from the cultural mycelium of Cordyceps sinensis. Biol. Pharm. Bull. 1993, 16, 1291–1293. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.K.; Walker, P.A.M.D.; Moore-Olufemi, S.D.; Sundaresan, A.; Kulkarni, A.D.; Andrassy, R.J. An Evidence-Based Review of a Lentinula edodes Mushroom Extract as Complementary Therapy in the Surgical Oncology Patient. J. Parenteral Enteral. Nutr. 2011, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Kim, K.H.; Choi, H.J.; Lee, D.S. Anti-diabetic activity of β-glucans and their enzymatically hydrolyzed oligosaccharides from Agaricus blazei. Biotechnol. Lett. 2005, 27, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Wauthier, V.; Waxman, D.J. Sex-specific early growth hormone response genes in rat liver. Mol. Endocrinol. 2008, 22, 1962–1974. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Behr, S.R.; Newman, R.K.; Newman, C.W. Comparative cholesterol-lowering effects of barley β-glucan and barley oil in golden Syrian hamsters. Nutr. Res. 1997, 17, 77–88. [Google Scholar] [CrossRef]
- Lorenzi, T.F. Manual Haematology, 4th ed.; Guanabara Koogan: São Paulo, Brazil, 2006. [Google Scholar]
- Gulye, J.V.; Camicas, J.Z.; Diouf, A.M. Ticks and blood parasites in Senegal (Sahlian zone). Rev. Elevage Med. Vet. Pays Trop. 1988, 40, 119–125. [Google Scholar]
- Dacie, J.V.; Lewis, S.M. Practical Haematology; Churchill Livingstone: London, UK, 1991. [Google Scholar]
Sample Availability: Samples of lasiodiplodan are available from the authors (Contact: Mário A. Alves da Cunha, E-Mail: [email protected]). |




| Treatment group a | Cholesterol (mg/dL) | HDL b (mg/dL) | LDL c (mg/dL) | VLDL d (mg/dL) | Triacylglycerol (mg/dL) |
|---|---|---|---|---|---|
| Male | |||||
| Control | 143 ± 14.2 | 37 ± 3.9 | 70 ± 14.0 | 36 ± 12.0 | 178 ± 61.0 |
| Lasiodiplodan | 133 ± 17.2 | 36 ± 9.0 | 61 ± 14.1 | 36 ± 10.0 | 180 ± 50.0 |
| Female | |||||
| Control | 100 ± 8.17 | 26 ± 4.8 | 42 ± 16.6 | 32 ± 12.4 | 161 ± 19.0 |
| Lasiodiplodan | 91 ± 17.11 | 20 ± 6.6 | 38 ± 16.7 | 33 ± 8.5 | 164 ± 64.6 |
| Leukogram | Control (Mean ± SD) a | Lasiodiplodan (Mean ± SD) a | ||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Total leukocytes (mm3) | 4.30 ± 0.20 | 4.00 ± 0.31 | 4.20 ± 0.12 | 4.10 ± 0.67 |
| Neutrophils (%) | 25.00 ± 1.58 | 25.50 ± 1.50 | 21.00 ± 2.21 | 21.87 ± 3.32 |
| Basophils (%) | 0.62 ± 0.18 | 1.12 ± 0.22 | 1.00 ± 0.18 | 1.42 ± 0.20 |
| Lymphocytes (%) | 71.12 ± 2.01 | 72.12 ± 2.30 | 75.50 ± 2.73 | 75.12 ± 3.18 |
| Monocytes (%) | 2.85 ± 0.91 | 2.95 ± 0.85 | 1.97 ± 0.45 | 3.00 ± 0.46 |
| Eosinophils (%) | 0.37 ± 0.18 | 0.75 ± 0.17 | 0.12 ± 0.08 | 0.12 ± 0.08 |
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Túrmina, J.A.; Carraro, E.; Alves da Cunha, M.A.; Dekker, R.F.H.; Barbosa, A.M.; Dos Santos, F.S.; Silva, L.A.; Malfatti, C.R.M. Toxicological Assessment of ?-(1à6)-Glucan (Lasiodiplodan) in Mice during a 28-Day Feeding Study by Gavage. Molecules 2012, 17, 14298-14309. https://doi.org/10.3390/molecules171214298
Túrmina JA, Carraro E, Alves da Cunha MA, Dekker RFH, Barbosa AM, Dos Santos FS, Silva LA, Malfatti CRM. Toxicological Assessment of ?-(1à6)-Glucan (Lasiodiplodan) in Mice during a 28-Day Feeding Study by Gavage. Molecules. 2012; 17(12):14298-14309. https://doi.org/10.3390/molecules171214298
Chicago/Turabian StyleTúrmina, Janaína A., Emerson Carraro, Mário A. Alves da Cunha, Robert F. H. Dekker, Aneli M. Barbosa, Fábio Seidel Dos Santos, Luiz A. Silva, and Carlos R. M. Malfatti. 2012. "Toxicological Assessment of ?-(1à6)-Glucan (Lasiodiplodan) in Mice during a 28-Day Feeding Study by Gavage" Molecules 17, no. 12: 14298-14309. https://doi.org/10.3390/molecules171214298
APA StyleTúrmina, J. A., Carraro, E., Alves da Cunha, M. A., Dekker, R. F. H., Barbosa, A. M., Dos Santos, F. S., Silva, L. A., & Malfatti, C. R. M. (2012). Toxicological Assessment of ?-(1à6)-Glucan (Lasiodiplodan) in Mice during a 28-Day Feeding Study by Gavage. Molecules, 17(12), 14298-14309. https://doi.org/10.3390/molecules171214298




