Computational Termochemistry Study of the C80 Isomers and Their Endo Lanthanum Complexes by Applying Homodesmotic and Isodesmic Reactions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Pristine Fullerene
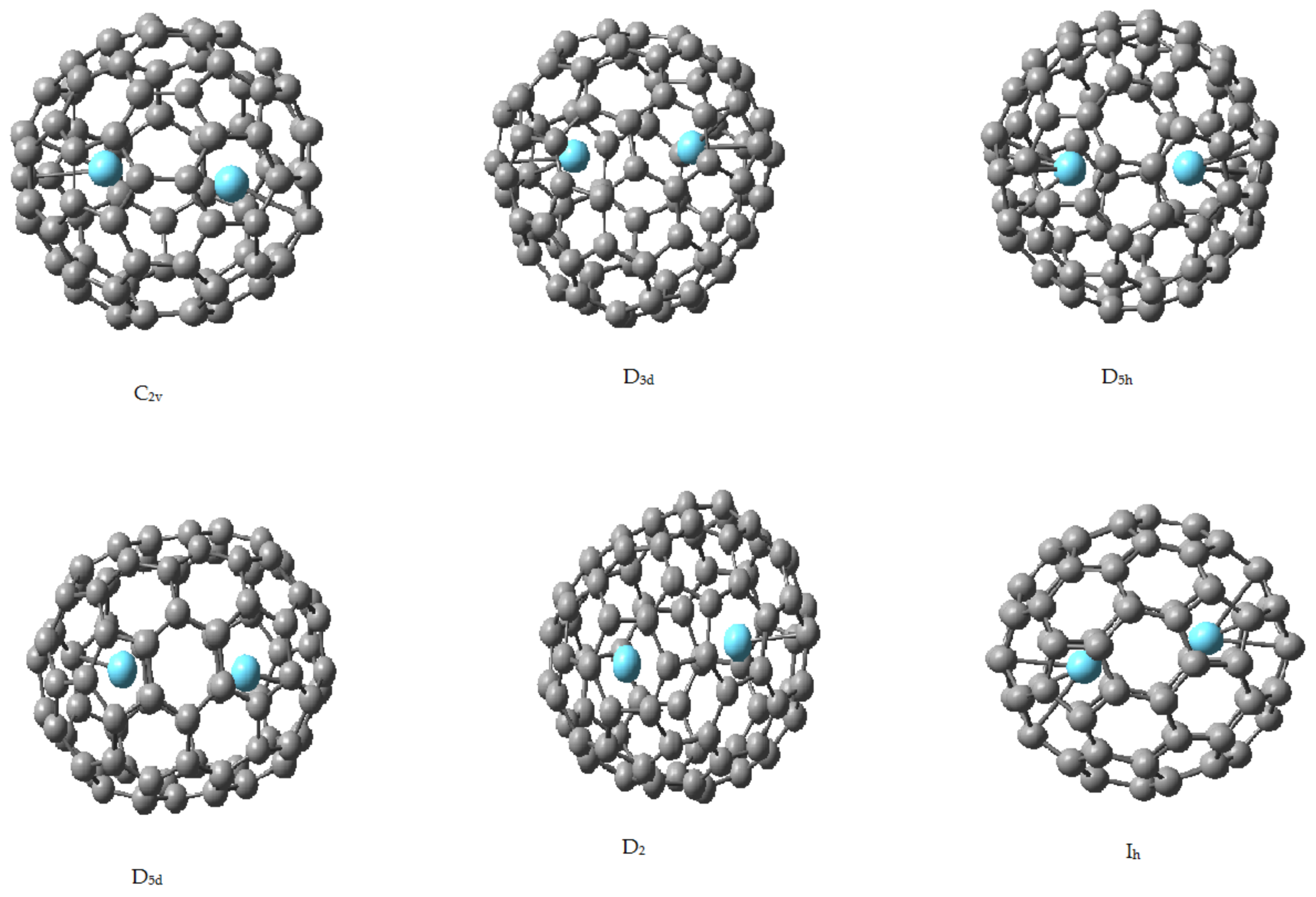
2.2. Endohedral Lanthanum Complexes
3. Experimental
4. Conclusions
Acknowledgements
References and Notes
- Kadish, K.M.; Ruoff, R.S. (Eds.) Fullerenes: Chemistry, Physics and Technology; Wiley-Interscience: New York, NY, USA, 2000. [Google Scholar]
- Yamada, M.; Akasaka, T.; Nagase, S. Endohedral metal atoms in pristine and functionalized fullerene cages. Acc. Chem. Res. 2010, 43, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Nikawa, H.; Nakahodo, T.; Tsuchiya, T.; Ishitsuka, M.O.; Maeda, Y.; Akasaka, T.; Toki, M.; Sawa, H.; Slanina, Z.; et al. Chemical understanding of a non-IPR Metallofullerene: Stabilization of encaged metals on fused-pentagon bonds in La2@C72. J. Am. Chem. Soc. 2008, 130, 9129–9136. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, H. Endohedrals. Rep. Prog. Phys. 2000, 63, 843–892. [Google Scholar] [CrossRef]
- Kroto, H.W. Determing the structure of frontier carbon materials. Nature 1987, 329, 529–532. [Google Scholar] [CrossRef]
- Sun, M.-L.; Slanina, Z.; Lee, S.-L.; Uhlik, F.; Adamowicz, L. Excited electronic states and relative stabilities of C80 isomers. Chem. Phys. Lett. 1995, 246, 66–71. [Google Scholar] [CrossRef]
- Heinnrich, F.H.; Michel, R.H.; Fischer, A.; Richard-Schneider, S.; Gilb, S.; Kappes, M.M.; Fuchs, D.; Bürk, M.; Kobayashi, K.; Nagase, S. Isolation and preparation of C80. Angew. Chem. Int. Ed. Engl. 1996, 35, 1732–1734. [Google Scholar] [CrossRef]
- Wang, C.-R.; Sugai, T.; Kai, T.; Tomiyama, T.; Shinohara, H. Production and isolation of C80 fullerene. Chem. Commun. 2000, 557–558. [Google Scholar] [CrossRef]
- Shustova, N.B.; Kuvychko, I.V.; Bolskar, R.D.; Seppelt, K.; Strauss, S.H.; Popov, A.A.; Boltalina, O.V. Trifluoromethyl derivatives of insoluble small HOMO-LUMO gap hollow higher fullerenes. J. Am. Chem. Soc. 2006, 128, 15793–15798. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Dunsch, L. Expanding the number of stable stable isomeric structures of the C80 cage. A new fullerene DY3N@C80. Chem. Eur. J. 2006, 12, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Duchamp, J.C.; Demortier, A.; Fletcher, K.R.; Dorn, D.; Iezzi, E.B.; Glass, T.; Dorn, H.C. An isomer of the endohedral matallofullerene Sc3N@C-80 with D5h symmetry. Chem. Phys. Lett. 2003, 375, 655–659. [Google Scholar] [CrossRef]
- Stevenson, S.; Rice, G.; Glass, T.; Harik, K.; Cromer, F.; Jordan, M.R.; Craft, J.; Hadju, E.; Bible, R.; Olmstead, M.M.; et al. Small band-gap endohedral metallofullerenes in high yield and purity. Nature 1999, 401, 55–57. [Google Scholar] [CrossRef]
- Kobayashi, K.; Nagase, S.; Akasaka, T. A theoretical study of C80 and La@C80. Chem. Phys. Lett. 1995, 245, 230–235. [Google Scholar] [CrossRef]
- Lu, X.; Akasaka, T.; Nagase, S. Rare Earth Metals Trapped inside Fullerenes-Endohedral Metallofullerenes. In Rare Earth Coordination Chemistry, From Basic to Applications; Huang, C.H., Ed.; John Wiley & Sons: Singapore, 2010. [Google Scholar]
- Rojas, A.; Martínez, M.; Amador, P.; Torres, L.A. Increasing Stability of the Fullerenes with the Number of Carbon atoms: The Experimental Evidence. J. Phys. Chem. B 2007, 111, 9031–9035. [Google Scholar] [CrossRef] [PubMed]
- Furche, F.; Alhrichs, R. Fullerene C80: Are there still more isomers? J. Chem. Phys. 2001, 114, 10362–10367. [Google Scholar] [CrossRef]
- Slanina, Z.; Lee, S.-L.; Uhlik, F.; Adamowicz, L.; Nagase, S. Excited Electronic States and Relative Stabilities of C80 Isomers. Int. J. Quantum Chem. 2006, 106, 2222–2228. [Google Scholar] [CrossRef]
- Aihara, J.-I.; Yoshida, M. Weighted HOMO-LUMO energy separations of properly closed-shell fullerene isomers. J. Mol. Graph. Model. 2001, 19, 194–198. [Google Scholar] [CrossRef]
- Cioslowski, J.; Rao, N.; Moncrieff, D. Standard enthalpies of formation of fullerenes and their dependence on structural motifs. J. Am. Chem. Soc. 2000, 122, 8265–8270. [Google Scholar] [CrossRef]
- Slanina, Z.; Zhao, X.; Kurita, N.; Gotoh, H.; Uhlík, F.; Rudziǹski, J.M.; Lee, K.H.; Adamowikz, L. Computing the relative gas-phase populations of C60 and C70: Beyond the traditional ΔH°f,298 scale. J. Mol. Graph. Model. 2001, 19, 216–221. [Google Scholar] [CrossRef]
- Nakamoto, K.; McKinney, M.A. Application of the correlation method to vibrational spectra of C-60 and other fullerenes. J. Chem. Educ. 2000, 77, 775–780. [Google Scholar] [CrossRef]
- Kamarás, K.; Iwasa, Y.; Forró, L. Infrared spectra of one and two dimensional fullerene polymer structures. RbC-60 and rhombohedral C-60. Phys. Rev. B 1997, 55, 10999–11002. [Google Scholar] [CrossRef]
- Klupp, G.; Kamarás, K. Following Jahn-Teller Distortions in Fulleride Salts by Optical Spectroscopy, The Jahn-Teller Effect. In Springer Series in Chemical Physics 97; Köppel, H., Yarkony, D.R., Barentzen, H., Eds.; Springer: New York, NY, USA, 2009; pp. 489–515. [Google Scholar]
- Fowler, P.F.; Manolopoulos, D.E. (Eds.) An Atlas of Fullerenes; Dover Publications: Mineola, NY, USA, 2006. [Google Scholar]
- Fowler, P.W.; Manolopoulos, D.E. Magic Numbers and Stable Structures for Fullerenes, Fullerides and Fullerenium Ions. Nature 1992, 355, 428–430. [Google Scholar] [CrossRef]
- Suzuki, T.; Maruyama, Y.; Kato, T.; Kikuchi, K.; Nakao, Y.; Achiba, Y.; Kobayashi, K.; Nagase, S. Electrochemistry and ab initio study of the dimetallofullerene La2@C80. Angew. Chem. Int. Ed. Engl. 1995, 34, 1094–1096. [Google Scholar] [CrossRef]
- Austin, S.J.; Baker, J.; Fowler, P.W.; Manolopoulos, D.E. Bond stretch isomerism and the fullerenes. J. Chem. Soc. Perkin Trans. 1994, 2, 2319–2323. [Google Scholar] [CrossRef]
- George, P.; Trachtman, M.; Bock, C.W.; Brett, A.M. Homodesmotic reactions for the assessment of stabilization energies in benzenoid and other conjugated cyclic hydrocarbons. J. Chem. Phys. Perkin Trans II 1976, 1222–1227. [Google Scholar] [CrossRef]
- George, P.; Trachtman, M.; Bock, C.W.; Brett, A.M. An alternative approach to the problem of assessing stabilization energies in cyclic conjugated hydrocarbons. Theoret. Chim. Acta (Berlin) 1975, 38, 121–129. [Google Scholar] [CrossRef]
- Salcedo, R.; Fomina, L. A homodesmotic reaction for fullerenes. Tetrahedron Lett. 2007, 48, 3949–3952. [Google Scholar] [CrossRef]
- Salcedo, R.; Fomina, L. A homodesmotic reaction for fullerenes, Corrigendum. Tetrahedron Lett. 2007, 48, 7731. [Google Scholar] [CrossRef]
- Salcedo, R. Fullerenocene. Polyhedron 2009, 28, 431–436. [Google Scholar] [CrossRef]
- Slayden, S.W.; Liebman, J.F. The Energetics of Aromatic Hydrocarbons: An Experimental Thermochemical Perspective. Chem. Rev. 2001, 101, 1541–1566. [Google Scholar] [CrossRef] [PubMed]
- Gun’ko, Y.K.; Hitchcock, P.B.; Lappert, M.F. Non-classic Organolanthanoid Metal Chemistry: [K([18]-crown-6)(η6-PhMe)2]X (X=[LnCpt3)2(μ-H)], [LnCp’’2)2(μ-η6:η6-PhMe]) from [LnCpX3], K and [18]-crown-6 in Toluene (Ln= Ln, Ce; Cpt=η5-CpC5H4SiMe2But; Cp’’=η5C5H3(SiMe3)2–1,3). Organometallics 2000, 19, 2832–2834. [Google Scholar] [CrossRef]
- Akasaka, T.; Nagase, S.; Kobayashi, K.; Wälchli, M.; Yamamoto, K.; Funasaka, H.; Kako, M.; Hoshino, T.; Erata, T. 13C and 139La NMR studies of La@C80: First Evidence for Circular Motion of Metal Atoms in Endohedral Dimetallofullerenes. Angew. Chem. Int. Ed. Engl. 1997, 36, 1643–1645. [Google Scholar] [CrossRef]
- Kobayashi, K.; Nagase, S.; Maeda, Y.; Wakahara, T.; Akasaka, T. La2@C80: Is the circular motion of two La atoms controllable by exohedral addition? Chem. Phys. Lett. 2003, 374, 562–566. [Google Scholar] [CrossRef]
- Reiher, M.; Hirsch, A. From rare gas atoms to fullerenes: Spherical aromaticity studied from the point of view of atomic structure Theory. Chem. Eur. J. 2003, 9, 5442–5452. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, H.; Yamada, T.; Cao, B.; Mizorigi, N.; Slanina, Z.; Tsuchiya, T.; Akasaka, T.; Yoza, K.; Nagase, S. Missing metallofullerene with C80 Cage. J. Am. Chem. Soc. 2009, 131, 10950–10954. [Google Scholar] [CrossRef] [PubMed]
- Bambirra, S.; Meetsma, A.; Hessen, B. Lanthanum tribenzyl complexes as convenient starting materials for organolanthanum chemistry. Organometallics 2006, 25, 3454–3462. [Google Scholar] [CrossRef]
- Popov, A.A. Metal-cage bonding, Molecular structure and vibrational spectra of endohedral fullerenes: Bridging experiment and theory. J. Comp. Theor. Nanosci. 2009, 6, 292–317. [Google Scholar] [CrossRef]
- Popov, A.A.; Dunsch, L. Bonding in endohedral metallofullerenes as studied by Quantum Theory of Atoms in Molecules. Chem. Eur. J. 2009, 15, 9707–9729. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Seki, S.; Luo, G.; Suzuki, M.; Lu, J.; Nagase, S.; Akasaka, T. Tunable Charge-Transport Properties of Ih-C80 Endohedral Metallofullerenes: Investigation of La2@C80, Sc3N@C80, and Sc3C2@C80. J. Am. Chem. Soc. 2012, 134, 11681–11686. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.A.; Avdoshenko, S.M.; Pendás, A.M.; Dunsch, L. Bonding between strongly repulsive metal atoms: an oxymoron made real in a confined space of endohedral metallofullerenes. Chem. Commun. 2012, 48, 8031–8050. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, K.; Larsson, J.A. Explanation of the different binding sites for Ce and La in M2@C80 (M= Ce, La)—A density functional theory prediction. J. Mat. Chem. 2008, 18, 3347–3351. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, Noncovalent interactions, Excited states, And transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, Thermochemical kinetics, Noncovalent interactions, EXCITED states, And transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 119, 525. [Google Scholar]
- Pantazis, D.A.; Neese, F. All electron scalar relativistic basis sets for lanthanides. J. Chem. Theor. Comput. 2009, 5, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Pantazis, D.A.; Chen, X.-Y.; Landis, C.R.; Neese, F. All electron scalar relativistic basis sets for third row transition metal atoms. J. Chem. Theor. Comput. 2008, 4, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.E.; Houk, K.N.; Scheleyer, P.V.R.; Allen, W.D.A. Hierarchy of Homodesmotic Reactions for Thermochemistry. J. Am. Chem. Soc. 2009, 131, 2547–2560. [Google Scholar] [CrossRef] [PubMed]
Samples Availability: Samples of the compounds are not available from the authors. |


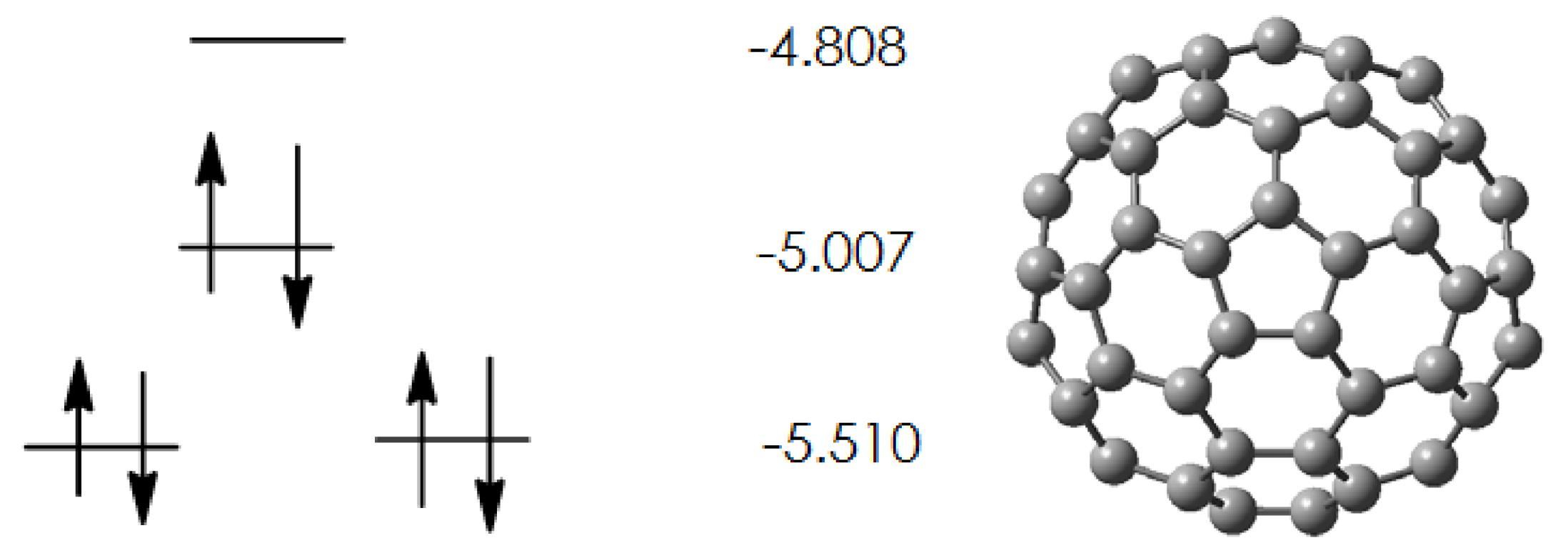







| Isomer | ∆H | Rel. energy |
|---|---|---|
| D2 | −153.75 | 0.0 |
| D5d | −152.37 | 3.83 |
| C2v | −146.23 | 5.94 |
| D3 | −145.26 | 8.76 |
| D5h | −144.34 | 11.25 |
| Ih | −128.03 | 20.98 |
| Isomer | ∆H (kcal/mol) | Rel. energy |
|---|---|---|
| Ih | 80.82 | 0.0 |
| D5h | 118.80 | 11.55 |
| C2v | 155.26 | 19.54 |
| D3 | 188.40 | 63.27 |
| D2 | 209.75 | 88.87 |
| D5d | 227.21 | 97.89 |
| Isomer | C-La | La-La |
|---|---|---|
| Ih | 2.470 | 3.710 |
| D5h | 2.527 | 3.735 |
| C2v | 2.550 | 3.748 |
| D3 | 2.561 | 3.928 |
| D2 | 2.570 | 4.005 |
| D5d | 2.584 | 4.150 |
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rios, C.; Salcedo, R. Computational Termochemistry Study of the C80 Isomers and Their Endo Lanthanum Complexes by Applying Homodesmotic and Isodesmic Reactions. Molecules 2012, 17, 14588-14601. https://doi.org/10.3390/molecules171214588
Rios C, Salcedo R. Computational Termochemistry Study of the C80 Isomers and Their Endo Lanthanum Complexes by Applying Homodesmotic and Isodesmic Reactions. Molecules. 2012; 17(12):14588-14601. https://doi.org/10.3390/molecules171214588
Chicago/Turabian StyleRios, Citlalli, and Roberto Salcedo. 2012. "Computational Termochemistry Study of the C80 Isomers and Their Endo Lanthanum Complexes by Applying Homodesmotic and Isodesmic Reactions" Molecules 17, no. 12: 14588-14601. https://doi.org/10.3390/molecules171214588