The Role of Water in Lanthanide-Catalyzed Carbon–Carbon Bond Formation
Abstract
:1. Introduction

2. Results and Discussion
 and
and  represent the measured decay rates in H2O and D2O, respectively; q represents the average water-coordination number; and α accounts for the influence of non-coordinated molecules on luminescence decay [19]. We found the average water-coordination numbers of the studied europium salts in H2O/THF mixtures ranging from 1 to 40% H2O in THF (v/v) to be between 3.2 and 8.6 water molecules (Figure 2, Table 1).
represent the measured decay rates in H2O and D2O, respectively; q represents the average water-coordination number; and α accounts for the influence of non-coordinated molecules on luminescence decay [19]. We found the average water-coordination numbers of the studied europium salts in H2O/THF mixtures ranging from 1 to 40% H2O in THF (v/v) to be between 3.2 and 8.6 water molecules (Figure 2, Table 1).

| (a) | (b) | |||
|---|---|---|---|---|
| % H2O in THF (v/v) | q | % H2O in THF (v/v) | q | |
| 1 | 5.8 ± 0.1 | 1 | 3.2 ± 0.02 | |
| 5 | 8.0 ± 0.1 * | 5 | 5.2 ± 0.04 | |
| 10 | 8.3 ± 0.03 * | 10 | 6.8 ± 0.03 | |
| 15 | 8.6 ± 0.02 | 15 | 7.2 ± 0.03 | |
| 20 | 8.6 ± 0.1 * | 20 | 7.2 ± 0.04 | |
| 25 | 8.6 ± 0.1 | 25 | 7.4 ± 0.04 | |
| 30 | 8.5 ± 0.04 * | 30 | 7.6 ± 0.1 | |
| 40 | 8.5 ± 0.01 * | 40 | 7.7 ± 0.01 |


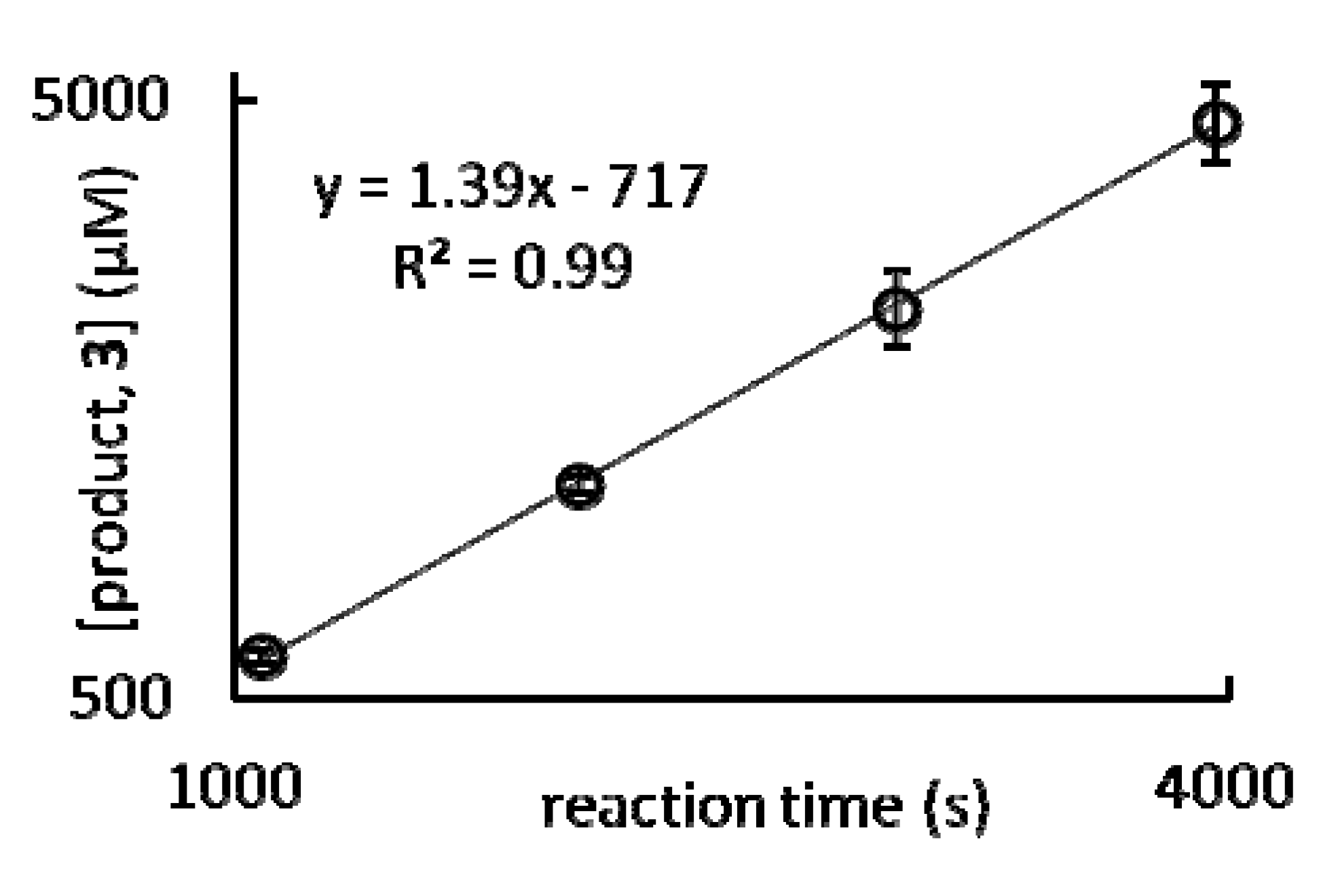
| (a) | (b) | |||
|---|---|---|---|---|
| % H2O in THF (v/v) | steady state reaction rate (μM s–1) | % H2O in THF (v/v) | steady state reaction rate (μM s–1) | |
| 0 | 0.29 ± 0.06 | 0 | nd | |
| 1 | 0.33 ± 0.03 | 1 | 0.008 ± 0.001 | |
| 5 | 1.0 ± 0.2 | 5 | 0.11 ± 0.02 | |
| 10 | 1.4 ± 0.2 | 10 | 0.33 ± 0.06 | |
| 15 | 1.5 ± 0.2 | 15 | 0.48 ± 0.04 | |
| 20 | nd | 20 | nd | |
| 25 | 1.45 ± 0.08 | 25 | 1.0 ± 0.2 | |
| 30 | nd | 30 | nd | |
| 40 | 1.3 ± 0.1 | 40 | 1.3 ± 0.2 |

3. Experimental Section
3.1. General
3.2. Mukaiyama Aldol Reaction Protocol
3.3. Luminescence-Decay Measurements
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Kobayashi, S.; Hachiya, I. Lanthanide triflates as water-tolerant Lewis acids. Activation of commercial formaldehyde solution and use in the aldol reaction of silyl enol ethers with aldehydes in aqueous media. J. Org. Chem. 1994, 59, 3590–3596. [Google Scholar] [CrossRef]
- Kobayashi, S.; Manabe, K. Green Lewis acid catalysis in organic synthesis. Pure Appl. Chem. 2000, 72, 1373–1380. [Google Scholar] [CrossRef]
- Hamada, T.; Manabe, K.; Ishikawa, S.; Nagayama, S.; Shiro, M.; Kobayashi, S. Catalytic asymmetric aldol reactions in aqueous media using chiral bis-pyridino-18-crown-6-rare earth metal triflate complexes. J. Am. Chem. Soc. 2003, 125, 2989–2996. [Google Scholar]
- Mei, Y.; Dissanayake, P.; Allen, M.J. A new class of ligands for aqueous, lanthanide-catalyzed enantioselective Mukaiyama aldol reactions. J. Am. Chem. Soc. 2010, 132, 12871–12873. [Google Scholar] [CrossRef]
- Mikami, K.; Kotera, O.; Motoyama, Y.; Sakaguchi, H. Lanthanide bis-trifluoromethanesulfony-lamides as a new type of asymmetric catalysts for hetero Diels-Alder reaction with Danishefsky’s diene in the presence of water. Synlett 1995, 975–977. [Google Scholar]
- Bernardelli, P.; Moradei, O.M.; Friedrich, D.; Yang, J.; Gallou, F.; Dyck, B.P.; Doskotch, R.W.; Lange, T.; Paquette, L.A. Total asymmetric synthesis of the putative structure of the cytotoxic diterpenoid (–)-sclerophytin A and of the authentic natural sclerophytins A and B. J. Am. Chem. Soc. 2001, 123, 9021–9032. [Google Scholar]
- Qian, C.; Huang, T. Glyoxylate-ene reaction catalyzed by Ln(OTf)3. Tetrahedron Lett. 1997, 38, 6721–6724. [Google Scholar] [CrossRef]
- Aspinall, H.C.; Greeves, N.; McIver, E.G. Ytterbium triflate catalysed allylation of aldehydes: An unusual benzoic acid induced acceleration. Tetrahedron Lett. 1998, 39, 9283–9286. [Google Scholar] [CrossRef]
- Kinsman, A.C.; Kerr, M.A. Highly selective Diels-Alder reactions of dienophiles with 1,3-cyclohexadiene mediated by Yb(OTf)3·2H2O and ultrahigh pressures. Org. Lett. 2000, 2, 3517–3520. [Google Scholar] [CrossRef]
- Crousse, B.; Bégué, J.-P.; Bonnet-Delpon, D. Synthesis of 2-CF3-tetrahydroquinoline and quinoline derivatives from CF3-N-Aryl-aldimine. J. Org. Chem. 2000, 65, 5009–5013. [Google Scholar] [CrossRef]
- Sanchez-Blanco, A.I.; Gothelf, K.V.; Jørgensen, K.A. Lanthanide-catalyzed endo- and enantioselective 1,3 dipolar cycloaddition reactions of nitrones with alkenes. Tetrahedron Lett. 1997, 38, 7923–7926. [Google Scholar] [CrossRef]
- Kawada, A.; Mitamura, S.; Kobayashi, S. Lanthanide trifluoromethanesulfonates as reusable catalysts: catalytic Friedel-Crafts acylation. Chem. Commun. 1993, 1157–1158. [Google Scholar]
- Kobayashi, S.; Ishitani, H.; Komiyama, S.; Oniciu, D.C.; Katritzky, A.R. A novel Mannich-type reaction: lanthanide triflate-catalyzed reactions of N-(α-Aminoalkyl)benzotriazoles with silyl enolates. Tetrahedron Lett. 1996, 37, 3731–3734. [Google Scholar]
- Ishitani, H.; Kobayashi, S. Catalytic asymmetric aza Diels-Alder reactions using a chiral lanthanide Lewis acid. Enantioselective synthesis of tetrahydroquinoline derivatives using a catalytic amount of a chiral source. Tetrahedron Lett. 1996, 37, 7357–7360. [Google Scholar] [CrossRef]
- Li, H.-J.; Tian, H.-Y.; Wu, Y.-C.; Chen, Y.-J.; Liu, L.; Wang, D.; Li, C.-J. Aqueous asymmetric Mukaiyama aldol reaction catalyzed by chiral gallium Lewis acid with Trost-type semi-crown ligands. Adv. Synth. Catal. 2005, 347, 1247–1256. [Google Scholar] [CrossRef]
- Ollevier, T.; Plancq, B. Highly enantioselective Mukaiyama aldol reaction in aqueous conditions using a chiral iron(II) bipyridine catalyst. Chem. Commun. 2012. [Google Scholar] [CrossRef]
- Cossy, C.; Helm, L.; Merbach, A.E. Oxygen-17 nuclear magnetic resonance kinetic study of water exchange on the lanthanide(III) aqua ions. Inorg. Chem. 1988, 27, 1973–1979. [Google Scholar] [CrossRef]
- Dissanayake, P.; Allen, M.J. Dynamic measurements of aqueous lanthanide triflate-catalyzed reactions using luminescence decay. J. Am. Chem. Soc. 2009, 131, 6342–6343. [Google Scholar] [CrossRef]
- Dissanayake, P.; Mei, Y.; Allen, M.J. Luminescence-decay as an easy-to-use tool for the study of lanthanide-containing catalysts in aqueous solutions. ACS Catal. 2011, 1, 1203–1212. [Google Scholar] [CrossRef]
- Horrocks, W.D., Jr.; Sudnick, D.R. Lanthanide ion probes of structure in biology. Laser-induced luminescence decay constants provide a direct measure of the number of metal-coordinated water molecules. J. Am. Chem. Soc. 1979, 101, 334–340. [Google Scholar] [CrossRef]
- Hwang, B.J. Induction model for the heterogeneously-catalyzed liquid-phase oxidation of aldehydes. Ind. Eng. Chem. Res. 1994, 33, 1897–1900. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Merbach, A.E.; Nielson, R.M. 139La NMR and quantitative FT-IR investigation of the interaction between Ln(III) ions and various anions in organic solvents. Inorg. Chim. Acta 1987, 139, 151–152. [Google Scholar] [CrossRef]
- Barge, A.; Cravotto, G.; Gianolio, E.; Fedeli, F. How to determine free Gd and free ligand in solution of Gd chelates. A technical note. Contrast Med. Mol. Imaging 2006, 1, 184–188. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Averill, D.J.; Dissanayake, P.; Allen, M.J. The Role of Water in Lanthanide-Catalyzed Carbon–Carbon Bond Formation. Molecules 2012, 17, 2073-2081. https://doi.org/10.3390/molecules17022073
Averill DJ, Dissanayake P, Allen MJ. The Role of Water in Lanthanide-Catalyzed Carbon–Carbon Bond Formation. Molecules. 2012; 17(2):2073-2081. https://doi.org/10.3390/molecules17022073
Chicago/Turabian StyleAverill, Derek J., Prabani Dissanayake, and Matthew J. Allen. 2012. "The Role of Water in Lanthanide-Catalyzed Carbon–Carbon Bond Formation" Molecules 17, no. 2: 2073-2081. https://doi.org/10.3390/molecules17022073





