Correlation Between Chemical Composition and Antibacterial Activity of Essential Oils from Fifteen Eucalyptus Species Growing in the Korbous and Jbel Abderrahman Arboreta (North East Tunisia)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition
| Compounds andabbreviations | KI a | Content in % | ||||||
|---|---|---|---|---|---|---|---|---|
| Eucalyptus species | ||||||||
| E. astc | E. cam | E. div | E. dun | E. fal | E. glo | E. gom | ||
| α-Pinene (α-pin) | 1053 | 21.3 ± 4.2 | - b | 3.3 ± 3.2 | 23.7 ± 4.5 | 6.0 ± 1.7 | 12.0 ± 3.0 | 0.7 ± 0.6 |
| Limonene (lim) | 1209 | 1.3 ± 0.6 | 0.3 ± 0.6 | 1.0 ± 0.0 | 2.0 ± 1.0 | 0.7 ± 0.6 | 2.3 ± 1.5 | - |
| β-Phellandrene (β-phe) | 1215 | - | - | - | 1.0 ± 1.7 | - | - | - |
| 1,8-Cineole (1,8-cin) | 1218 | 43.7 ± 4.9 | 3.7 ± 2.1 | 37.0 ± 12.1 | 44.7 ± 2.1 | 30.7 ± 4.0 | 53.7 ± 3.2 | 6.3 ± 2.1 |
| p-Cymene (p-cym) | 1282 | 1.0 ± 0.0 | 11.7 ± 4.0 | 0.7 ± 0.6 | 1.0 ± 0.0 | - | 1.0 ± 0.0 | 3.0 ± 2.0 |
| Pinocarvone (pin) | 1590 | 1.7 ± 0.6 | - | 1.0 ± 1.0 | 1.3 ± 0.6 | 4.0 ± 0.0 | 1.7 ± 1.2 | 3.3 ± 1.5 |
| Terpinene-4-ol (ter-4-ol) | 1618 | - | 2.0 ± 0.0 | 0.7 ± 0.6 | - | - | - | 3.7 ± 1.5 |
| Aromadendrene (aro) | 1625 | 3.0 ± 1.0 | 0.3 ± 0.6 | 3.0 ± 2.0 | 1.3 ± 0.6 | 2.0 ± 0.0 | 3.7 ± 2.1 | 1.0 ± 0.0 |
| trans-Pinocarveol (tr-pin) | 1675 | 7.7 ± 2.3 | - | 7.0 ± 4.6 | 3.7 ± 2.1 | 26.0 ± 0.0 | 3.7 ± 1.5 | 12.3 ± 5.1 |
| Cryptone (cry) | 1695 | - | 12.7 ± 1.2 | - | - | - | - | - |
| α-Terpinol (α-ter) | 1713 | 1.3 ± 0.6 | - | 2.7 ± 2.1 | 1.3 ± 0.6 | 2.0 ± 0.0 | 3.3 ± 2.1 | 7.3 ± 2.3 |
| Borneol (bor) | 1720 | - | - | 0.7 ± 0.6 | - | 1.0 ± 0.0 | - | 2.0 ± 0.0 |
| Phellandral (phe) | 1747 | - | 3.7 ± 0.6 | - | - | - | - | - |
| Globulol (glo) | 2103 | 5.7 ± 1.2 | 1.0 ± 1.0 | 6.3 ± 5.5 | 4.3 ± 0.6 | 7.0 ± 1.7 | 4.7 ± 1.2 | 7.7 ± 1.2 |
| Viridiflorol (vir) | 2113 | 1.0 ± 0.0 | 1.0 ± 0.0 | 6.7 ± 8.1 | 1.7 ± 1.2 | 1.7 ± 1.2 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| Spathulenol (spa) | 2151 | 1.0 ± 0.0 | 28.0 ± 7.9 | 0.3 ± 0.6 | 1.7 ± 1.2 | 1.3 ± 0.6 | - | 1.0 ± 0.0 |
| Thymol (thy) | 2172 | - | 1.0 ± 0.0 | - | - | - | - | - |
| Isospathulenol (iso) | 2259 | - | - | - | - | - | 2.0 ± 3.5 | - |
| Compounds andabbreviations | KI a | Content in % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Eucalyptus species | |||||||||
| E. kit | E. leh | E. leu | E. mac | E. pla | E. pol | E. pop | E. rud | ||
| α-Pinene | 1053 | 9.7 ± 8.0 | 17.7 ± 7.2 | 7.7 ± 2.3 | 1.0 ± 0.0 | 9.3 ± 0.6 | 1.5 ± 0.7 | 2.0 ± 1.0 | 0.7 ± 0.6 |
| Limonene (lim) | 1209 | 1.0 ± 1.0 | 4.3 ± 0.6 | 2.3 ± 0.6 | 3.5 ± 0.7 | 0.3 ± 0.6 | 1.5 ± 0.7 | 1.3 ± 0.6 | 0.7 ± 0.6 |
| β-Phellandrene (β-phe) | 1215 | - | - | - | - | - | - | - | 7.3 ± 3.1 |
| 1,8-Cineole (1,8-cin) | 1218 | 4.7 ± 3.1 | 57.0 ± 4.4 | 59.3 ± 10.0 | 34.5 ± 2.1 | 22.7 ± 4.7 | 58.0 ± 12.07 | 47.0 ± 9.2 | 2.3 ± 2.1 |
| p-Cymene (p-cym) | 1282 | 6.7 ± 8.1 | 2.0 ± 0.0 | 3.0 ± 3.5 | 1.0 ± 0.0 | 7.7 ± 4.0 | 2.5 ± 2.1 | 0.7 ± 0.6 | 16.7 ± 1.5 |
| Pinocarvone (pin) | 1590 | 4.0 ± 1.7 | - | 1.0 ± 0.0 | - | 2.3 ± 0.6 | 0.5 ± .0.7 | 1.0 ± 1.0 | - |
| Terpinene-4-ol (ter-4-ol) | 1618 | 0.3 ± 0.6 | - | - | 1.0 ± 0.0 | 1.3 ± 0.6 | 0.5 ± 0.7 | 0.7 ± 0.6 | 2.0 ± 0.0 |
| Aromadendrene (aro) | 1625 | 1.3 ± 0.6 | - | 2.3 ± 1.5 | - | 1.0 ± 1.0 | - | 2.3 ± 1.2 | 0.7 ± 0.6 |
| trans-Pinocarveol (tr-pin) | 1675 | 21.7 ± 10.0 | 1.0 ± 0.0 | 4.7 ± 1.2 | - | 8.3 ± 2.5 | 1.5 ± 0.7 | 6.7 ± 5.5 | - |
| Cryptone (cry) | 1695 | - | - | - | - | - | 1.5 ± 2.1 | - | 7.0 ± 3.5 |
| α-Terpinol (α-ter) | 1713 | 4.7 ± 1.2 | 8.7 ± 2.3 | 1.7 ± 0.6 | 1.0 ± 0.0 | 1.3 ± 0.6 | 6.5 ± 0.7 | 2.7 ± 0.6 | 1.0 ± 0.0 |
| Borneol (bor) | 1720 | 4.7 ± 0.6 | 0.7 ± 0.6 | - | - | - | - | 0.7 ± 1.2 | - |
| Phellandral (phe) | 1747 | 0.3 ± 0.6 | - | - | - | - | 1.0 ± 0.0 | - | 4.3 ± 0.6 |
| Globulol (glo) | 2103 | 9.0 ± 7.8 | 0.7 ± 0.6 | 6.0 ± 2.6 | 2.0 ± 0.0 | 6.3 ± 2.1 | 2.0 ± 0.0 | 12.7 ± 1.5 | 0.7 ± 0.6 |
| Viridiflorol (vir) | 2113 | 1.3 ± 0.6 | - | 0.7 ± 0.6 | - | 3.7 ± 1.5 | 1.0 ± 0.0 | 2.0 ± 0.0 | - |
| Spathulenol (spa) | 2151 | 1.7 ± 0.6 | 1.0 ± 0.0 | - | 1.0 ± 0.0 | 11.0 ± 6.0 | 4.5 ± 3.5 | - | 19.7 ± 4.7 |
| Thymol (thy) | 2172 | - | - | - | 1.5 ± 0.7 | 0.3 ± 0.6 | - | - | 3.7 ± 2.5 |
| Isospathulenol (iso) | 2259 | - | - | - | - | 1.3 ± 0.6 | 0.5 ± 0.7 | - | 1.3 ± 0.6 |
2.2. Principal Components Analysis (PCA) and Hierarchical Cluster Analysis (HCA)
 ) and the compounds (
) and the compounds (  ), see Table 1.
), see Table 1.
 ) and the compounds (
) and the compounds (  ), see Table 1.
), see Table 1.
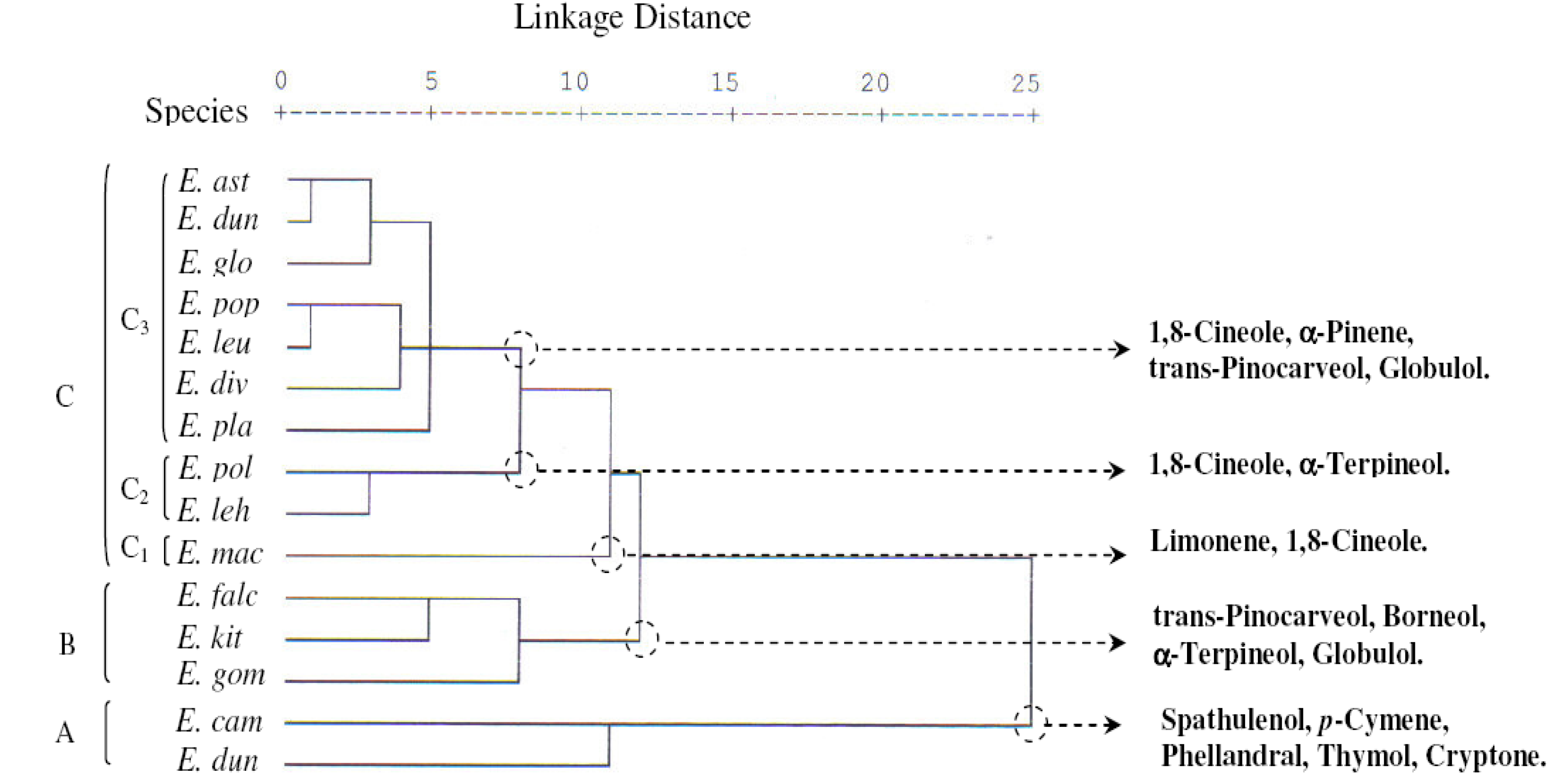
 ) see Table 1.
) see Table 1.
 ) see Table 1.
) see Table 1.

2.3. Antibacterial Activity
| Microorganisms | Inhibition zone diameters ♣ | |||||
|---|---|---|---|---|---|---|
| Essential oils | ||||||
| E. ast | E. cam | E. div | E. dun | E. fal | E. glo | |
| Gram+ | ||||||
| Enterococcus faecalis | 9.0 ± 1.0 a° | 9.0 ± 1.0 a | 9.3 ± 0.6 ab | 8.7 ± 2.1 a | 10.3 ± 2.1 abc | 8.7 ± 0.6 a |
| Staphylococcus aureus | 9.3 ± 1.5 abcd | 11.7 ± 0.6 de | 8.3 ± 2.5 ab | 8.0 ± 0.0 ab | 11.3 ± 3.8 cde | 9.0 ± 0.0 abcd |
| Gram− | ||||||
| Escherichia coli | 8.3 ± 0.6 bcd | 7.3 ± 0.6 abc | 8.3 ± 0.6 bcd | 7.0 ± 1.0 ab | 8.0 ± 2.0 bc | 8.0 ± 0.0 bc |
| Pseudomonas aeruginosa | 7.3 ± 0.6 ab | 7.0 ± 0.0 ab | 7.3 ± 0.6 ab | 7.0 ± 0.0 ab | 6.0 ± 0.0 a | 8.7 ± 0.6 b |
| Microorganisms | Inhibition zone diameters | ||||
|---|---|---|---|---|---|
| Essential oils | |||||
| E. gom | E. kit | E. leh | E. leu | E. mac | |
| Gram+ | |||||
| Enterococcusfaecalis | 8.3 ± 0.6 a | 8.3 ± 0.6 a | 8.3 ± 0.6 a | 8.3 ± 2.3 a | 8.5 ± 0.7 a |
| Staphylococcusaureus | 7.3 ± 0.6 a | 10.3 ± 1.5 bcd | 12.7 ± 0.0 e | 7.0 ± 0.0 a | 9.0 ± 0.0 abcd |
| Gram− | |||||
| Escherichia coli | 6.0 ± 0.0 a | 8.0 ± 1.7 bc | 10.0 ± 0.0 d | 6.7 ± 0.6 ab | 7.0 ± 0.0 ab |
| Pseudomonas aeruginosa | 6.0 ± 0.0 a | 7.7 ± 1.2 ab | 10.3 ± 0.6 c | 8.0 ± 1.0 b | 7.0 ± 0.0 ab |
| Microorganisms | Inhibition zone diameters | ||||
|---|---|---|---|---|---|
| Essential oils | |||||
| E. pla | E. pol | E. pop | E. rud | Gen ° | |
| Gram+ | |||||
| Enterococcus faecalis | 12.7 ± 5.5 cd | 11.1 ± 1.4 abc | 9.0 ± 1.0 a | 7.3 ± 1.2 a | 13.0 ± 1.0 d |
| Staphylococcusaureus | 8.0 ± 0.0 ab | 11.5 ± 2.1 cde | 9.3 ± 1.2 abcd | 8.7 ± 1.2 abc | 29.3 ± 1.2f |
| Gram− | |||||
| Escherichia coli | 7.3 ± 0.0 ab | 9.0 ± 0.0 cd | 8.3 ± 1.5 bcd | 7.0 ± 0.0 ab | 20.0 ± 1.0 e |
| Pseudomonas aeruginosa | 8.7 ± 0.6 b | 7.5 ± 0.7 ab | 8.7 ± 2.1 b | 6.0 ± 0.0 a | 14.3 ± 1.5 e |
3. Experimental
3.1. Plant Materials
3.2. Extraction of Essential Oils
3.3. Chemical Analysis
3.3.1. Gas Chromatography Analysis
3.3.2. Gas-Chromatography-Mass-Spectrometry Analysis
3.3.3. Compound Identification
3.4. Antibacterial Testing
3.5. Statistical Analysis
4. Conclusions
Acknowledgements
References and Notes
- Ogunwande, I.A.; Olawore, N.O.; Adeleke, K.A.; Konig, W.A. Chemical composition of the essential oils from the leaves of three Eucalyptus species growing in Nigeria. J. Essent. Oil Res. 2003, 15, 297–301. [Google Scholar] [CrossRef]
- Pino, J.A.; Marbot, R.; Quert, R.; Garcia, H. Study of essential oils of Eucalyptus resinifera Smith, E. tereticornis Smith and Corymbia maculata (Hook.) KD Hill & LAS Johnson, grown in Cuba. Flavour Frag. J. 2002, 17, 1–14. [Google Scholar] [CrossRef]
- Takahashi, T.; Kokubo, R.; Sakaino, M. Antimicrobial activities of Eucalyptus leaf extracts and flavonoids from Eucalyptus maculata. Lett. Appl. Microbiol. 2004, 39, 60–64. [Google Scholar] [CrossRef]
- Tepe, B.; Daferera, D.; Sökmen, M.; Polissiou, M.; Sökmen, A. In vitro antimicrobial and antioxidant activities of the essential oils and various extracts of Thymus eigii M Zohary et PH Davis. J. Agric. Food Chem. 2004, 52, 1132–1137. [Google Scholar] [CrossRef]
- Shuenzel, K.M.; Harisson, M.A. Microbial antagonists of food borne pathogen on fresh minimally processed vegetables. J. Food Protect. 2002, 65, 1909–1915. [Google Scholar]
- Cimanga, K.; Kambu, K.; Tona, L.; Apers, S.; De Bruyne, T.; Hermans, N.; Totté, J.; Pieters, L.; Vlietinck, A.J. Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. J. Ethnopharmacol. 2002, 79, 213–220. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Antimicrobial action of essential oil vapours and negative air ions against Pseudomonas fluorescens. Int. J. Food Microbiol. 2010, 143, 205–210. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Antimicrobial potential and chemical composition of Eucalyptus globulus oil in liquid and vapour phase against food spoilage microorganisms. Food Chem. 2011, 126, 228–235. [Google Scholar] [CrossRef]
- Moreira, M.R.; Ponce, A.G.; del Valle, C.E.; Roura, R.I. Inhibitory parameters of essential oils to reduce a foodborne pathogen. Lebensmittel-Wissenschaft und-Technology 2005, 38, 565–570. [Google Scholar]
- Low, D.; Rawal, B.D.; Griffin, W.J. Antibacterial action of the essential oils of some Australian Myrtaceae with special references to the activity of chromatographic fractions of oil of Eucalyptus citriodora. Planta Med. 1974, 26, 184–189. [Google Scholar] [CrossRef]
- Khouja, M.L.; Khaldi, A.; Rejeb, M.N. Results of the Eucalyptus Introduction Trials in Tunisia. In Proceedings of the International Conference of the Eucalyptus in the Mediterranean Basin: Perspectives and New Utilization, Taormina, Italy, 15-19 October 2000; Cannata, F., Ed.; Centro Promozione Pubblicita: Florence, Italy.
- Elaissi, A.; Chraif, I.; Bannour, F.; Farhat, F.; Ben Salah, M.; Chemli, R.; Khouja, M.L. Contribution to the qualitative and quantitative study of seven Eucalyptus species essential oil harvested of Hajeb’s Layoun arboreta (Tunisia). J. Essent. Oil Bear. Pl. 2007, 10, 15–25. [Google Scholar]
- Elaissi, A.; Medini, H.; Marzouki, H.; Khouja, M.L.; Lyenne, F.; Chemli, R.; Harzallah-Skhiri, F. Variation in volatile leaf oils of twelve Eucalyptus species harvested from Hajeb Layoun arboreta (Tunisia). Chem. Biodivers. 2010a, 7, 705–716. [Google Scholar] [CrossRef]
- Elaissi, A.; Marzouki, H.; Medini, H.; Khouja, M.L.; Farhat, F.; Lyenne, F.; Harzallah-Skhiri, F.; Chemli, R. Variation in volatile leaf oils of thirteen Eucalyptus species harvested from Souinet arboreta (Tunisia). Flav. Chem. Biodivers. 2010, 7, 909–921. [Google Scholar] [CrossRef]
- Elaissi, A.; Medini, H.; Khouja, M.L.; Monique, S.; Lyenne, F.; Farhat, F.; Chemli, R.; Harzallah-Skhiri, F. Variation in volatile leaf oils of eleven Eucalyptus species harvested from Korbous arboreta (Tunisia). Chem. Biodivers. 2010c, 7, 1841–1854. [Google Scholar] [CrossRef]
- Elaissi, A.; Medini, H.; Khouja, M.L.; Simmonds, M.; Lynen, F.; Farhat, F.; Chemli, R.; Harzallah-Skhiri, F. Variation in volatile leaf oils of five Eucalyptus species harvested from Jbel Abderrahman (Tunisia). Chem. Biodivers. 2011a, 8, 352–361. [Google Scholar]
- Elaissi, A.; Medini, H.; Simmonds, M.; Lynen, F.; Farhat, F.; Chemli, R.; Harzallah-Skhiri, F.; Khouja, M.L. Variation in volatile leaf oils of seven Eucalyptus Species harvested from Zerniza aroboreta (Tunisia). Chem. Biodivers. 2011b, 8, 362–376. [Google Scholar] [CrossRef]
- Schroeder, M.P.; Messing, A.M. Methods for comparing the antibacterial activity of essential oils and other aqueous insoluble compounds. Bull. Nat. Formulary Comm. 1949, 17, 213–218. [Google Scholar]
- Duraffourd, C.; Lapraz, J.C. Cuadernos de Fitoterapia Clínica; Ed. Masson: Mexico City, Mexico, 1986. [Google Scholar]
- Ponce, A.G.; Fritz, R.; del Valle, C.E.; Roura, S.I. Antimicrobial activity of essential oils on native microbial population of organic Swiss Chard. Lebensmitte-Wissencschaft und-Technology 2003, 36, 679–684. [Google Scholar]
- Carson, C.F.; Riley, T.V. Non-antibiotic therapies for infectious diseases. Commun. Dis. Intell. 2003, 27, 144–147. [Google Scholar]
- Dellacassa, E.; Menendez, P.; Moyna, P. Antimicrobial activity of Eucalyptus essential oils. Fitoterapia 1989, 60, 545–546. [Google Scholar]
- Zakarya, D.; Fkhih-Tetouani, S.; Hajji, F. Chemical composition-antimicrobial activity relationship of Eucalyptus essential oils. Plantes Médicinales et Phytothérapie 1993, 26, 331–339. [Google Scholar]
- Chalchat, J.C.; Garry, R.P.; Menut, C.; Lamaty, G.; Malhuret, R. Correlation between chemical composition and antimicrobial activity. VI. Activity of some African essential oils. J. Essent. Oil Res. 1997, 9, 67–75. [Google Scholar] [CrossRef]
- Essawi, T.; Sourour, M. Screening of some Palestinian medicinal plants for antibacterial activity. J. Ethnopharmacol. 2000, 70, 343–349. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Paster, N.; Menasherow, M.; Ravid, U.; Juven, B. Antifungal activity of oregano and thyme essential oils applied as fumigants against fungi attacking stored grain. J. Food Protect. 1995, 58, 81–85. [Google Scholar]
- Wiley Registry of Mass Spectral Data/NIST Spectral Data/CD Rom, 7th ed; John Wiley & Sons: New York, NY, USA, 1998.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Elaissi, A.; Rouis, Z.; Mabrouk, S.; Salah, K.B.H.; Aouni, M.; Khouja, M.L.; Farhat, F.; Chemli, R.; Harzallah-Skhiri, F. Correlation Between Chemical Composition and Antibacterial Activity of Essential Oils from Fifteen Eucalyptus Species Growing in the Korbous and Jbel Abderrahman Arboreta (North East Tunisia). Molecules 2012, 17, 3044-3057. https://doi.org/10.3390/molecules17033044
Elaissi A, Rouis Z, Mabrouk S, Salah KBH, Aouni M, Khouja ML, Farhat F, Chemli R, Harzallah-Skhiri F. Correlation Between Chemical Composition and Antibacterial Activity of Essential Oils from Fifteen Eucalyptus Species Growing in the Korbous and Jbel Abderrahman Arboreta (North East Tunisia). Molecules. 2012; 17(3):3044-3057. https://doi.org/10.3390/molecules17033044
Chicago/Turabian StyleElaissi, Ameur, Zyed Rouis, Samia Mabrouk, Karima Bel Haj Salah, Mahjoub Aouni, Mohamed Larbi Khouja, Farhat Farhat, Rachid Chemli, and Fethia Harzallah-Skhiri. 2012. "Correlation Between Chemical Composition and Antibacterial Activity of Essential Oils from Fifteen Eucalyptus Species Growing in the Korbous and Jbel Abderrahman Arboreta (North East Tunisia)" Molecules 17, no. 3: 3044-3057. https://doi.org/10.3390/molecules17033044




