Synthesis and Structural Determination of Novel 5-Arylidene-3-N(2-alkyloxyaryl)-2-thioxothiazolidin-4-ones
Abstract
:1. Introduction

2. Results and Discussion





3. Experimental
3.1. General
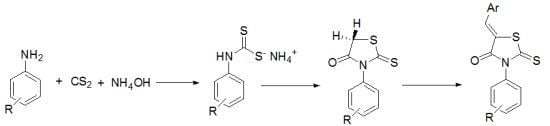
3.2. Typical Procedure for the Preparation of N-Arylthiazolidin-4ones e, f
3.3. Typical Procedure for the Preparation of 5-Arylidenethiazolidin-4-ones 1g–10g
4. Conclusions
Acknowledgments
References
- Dogan, I.; Burgemeister, T.; Icli, S.; Mannschreck, A. Synthesis and NMR studies of chiral 4-oxazolidinones and rhodanines. Tetrahedron 1992, 48, 7157–7164. [Google Scholar] [CrossRef]
- Karatas, M.; Koni, S.; Dogan, I. Chiral N-(o-aryl)-thiazolidinediones: Synthesis from rhodanines and investigation on rotational enantiomers by NMR spectroscopy. Can. J. Chem. 1998, 76, 255–259. [Google Scholar]
- Erol, S.; Dogan, I. Axially chiral 2-arylimino-3-aryl-thiazolidine-4-one derivatives: Enantiomeric separation and determination of racemization barriers by chiral HPLC. J. Org. Chem. 2007, 72, 2494–2500. [Google Scholar] [CrossRef]
- Kasmi-Mir, S.; Djafri, A.; Paquin, L.; Hamelin, J.; Rahmouni, M. One-pot synthesis of 5-aylidene-2-imino-4-thiazolidinones under microwave irradiation. Molecules 2006, 11, 597–602. [Google Scholar] [CrossRef]
- Kasmi-Mir, S.; Djafri, A.; Paquin, L.; Hamelin, J.; Bazureau, J.P.; Rahmouni, M. Synthesis of new rhodacyanines analogous to MKT-077 under microwave irradiation. Synth. Commun. 2007, 37, 4017–4034. [Google Scholar] [CrossRef]
- Verma, A.; Saraf, S.K. 4-Thiazolidinone-A biologically active scaffold. Eur.J. Med. Chem. 2008, 43, 897–905. [Google Scholar] [CrossRef]
- Prabhakar, Y.S.; Solomon, V.R.; Gupta, M.K.; Katti, S.B. QSAR studies on thiazolidines: A biologically privileged scaffold. Top. Heterocycl. Chem. 2006, 4, 161–249. [Google Scholar] [CrossRef]
- Havrylyuk, D.; Zimenkovsky, B.; Vasylenko, O.; Zaprutko, L.; Lesyk, R. Synthesis of novel thiazolone-based compounds containing pyrazoline moiety and evaluation of their anticancer activity. Eur. J. Med. Chem. 2009, 44, 1396–1404. [Google Scholar] [CrossRef]
- Joy, J.M.; Jacob, N.; Kutty, G.N. Evaluation of hypoglycemic effects of 4-thiazolidinones. Indian Drugs 2005, 42, 47–51. [Google Scholar]
- Zhou, H.; Wu, S.; Zhai, S.; Liu, A.; Sun, Y.; Li, R.; Zhang, Y.; Ekins, S.; Swaan, P.W.; Fang, B.; et al. Design, synthesis, cytoselective toxicity, structure-activity relationships, and pharmacophore of thiazolidinone derivatives targeting drug-resistant lung cancer cells. J. Med. Chem. 2008, 51, 1242–1251. [Google Scholar] [CrossRef]
- Smokal, V.; Derkowska, B.; Czaplicki, R.; Krupka, O.; Kolendo, A.; Sahraoui, B. Nonlinear optical properties of thiazolidinone derivatives. Opt. Mater. 2009, 31, 554–557. [Google Scholar] [CrossRef] [Green Version]
- Omar, K.; Geronikaki, A.; Zoumpoulakis, P.; Camoutsis, C.; Soković, M.; Cirić, A.; Glamoclija, J. Novel 4-thiazolidinone derivatives as potential antifungal and antibacterial drugs. Bioorg. Med. Chem. 2010, 18, 426–432. [Google Scholar] [CrossRef]
- Yapi, A.S.; Toumi, L.; Lare, Y.; Soto, G.M.; Cattin, L.; Toubal, K.; Djafri, A.; Morsli, M.; Khelil, A.; Del Valle, M.A.; et al. On the influence of the exciton-blocking layer on the organic multilayer cells properties. Eur. Phys. J. Appl. Phys. 2010, 50, 30403:1–30403:8. [Google Scholar]
- Vicini, P.; Geronikaki, A.; Incerti, M.; Zani, F.; Dearden, J.; Hewitt, M. 2-Heteroarylimino-5-benzylidene-4-thiazolidinones analogues of 2-thiazolylimino-5-benzylidene-4-thiazolidinones with antimicrobial activity: Synthesis and structure-activity relationship. Bioorg. Med. Chem. 2008, 16, 3714–3724. [Google Scholar] [CrossRef]
- Benhalima, N.; Toubal, K.; Chouaih, A.; Chita, G.; Maggi, S.; Djafri, A.; Hamzaoui, F. Synthesis and molecular structure investigation by DFT and X-Ray diffraction of ARNO. J. Chem. Crystallogr. 2011, 41, 1729–1736. [Google Scholar] [CrossRef]
- Sample Availability: Samples of compounds e,f and 1g–10g are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Toubal, K.; Djafri, A.; Chouaih, A.; Talbi, A. Synthesis and Structural Determination of Novel 5-Arylidene-3-N(2-alkyloxyaryl)-2-thioxothiazolidin-4-ones. Molecules 2012, 17, 3501-3509. https://doi.org/10.3390/molecules17033501
Toubal K, Djafri A, Chouaih A, Talbi A. Synthesis and Structural Determination of Novel 5-Arylidene-3-N(2-alkyloxyaryl)-2-thioxothiazolidin-4-ones. Molecules. 2012; 17(3):3501-3509. https://doi.org/10.3390/molecules17033501
Chicago/Turabian StyleToubal, Khaled, Ayada Djafri, Abdelkader Chouaih, and Abdou Talbi. 2012. "Synthesis and Structural Determination of Novel 5-Arylidene-3-N(2-alkyloxyaryl)-2-thioxothiazolidin-4-ones" Molecules 17, no. 3: 3501-3509. https://doi.org/10.3390/molecules17033501