Superconducting Fullerene Nanowhiskers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Observation of Morphologies

2.2. Superconducting Properties and X-ray Diffraction Patterns



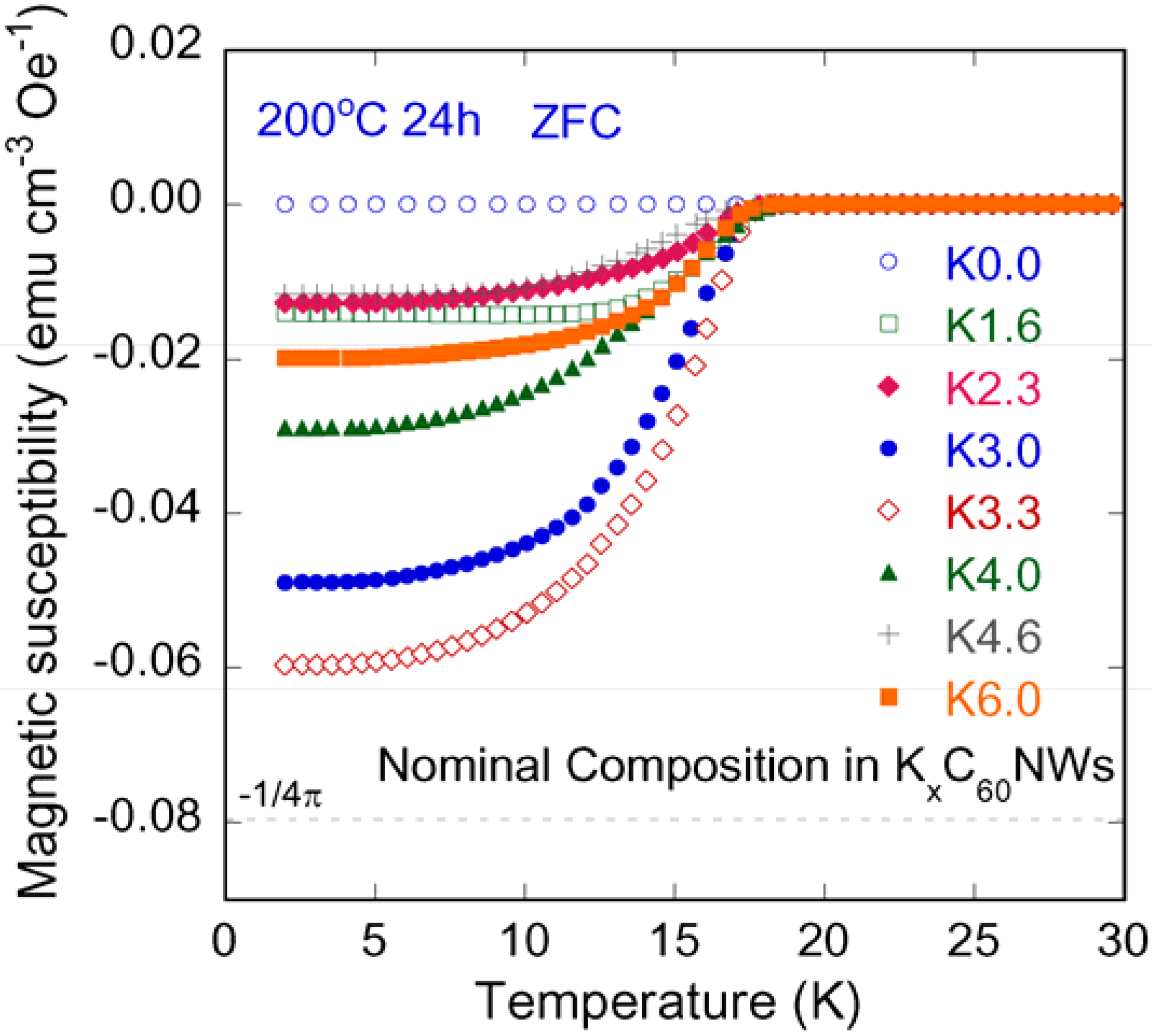
2.3. K-Compositional Dependence of Shielding Volume Fraction in KxC60NWs

3. Experimental
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the compounds, K-doped fullerene nanowhiskers, are not available from the authors.
References and Notes
- Kroto, H.W.; Hearth, L.D.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Hebard, A.F.; Rosseinsky, M.J.; Haddon, R.C.; Murphy, D.W.; Glarum, S.H.; Palstra, T.T.M.; Ramirez, A.P.; Kortan, A.R. Superconductivity at 18 K in potassium-doped C60. Nature 1991, 350, 600–601. [Google Scholar] [CrossRef]
- Murphy, D.W.; Rosseinsky, M.J.; Haddon, R.C.; Ramirez, A.P.; Hebard, A.F.; Tycko, R.; Fleming, R.M.; Dabbagh, G. Superconductivity in alkali metal fullerides. Physica C 1991, 185–189, 403–408. [Google Scholar]
- Miyazawa, K.; Kuwasaki, Y.; Obayashi, A.; Kuwabara, M. C60 nanowhiskers formed by the liquid-liquid interfacial precipitation method. J. Mater. Res. 2002, 17, 83–88. [Google Scholar] [CrossRef]
- Miyazawa, K.; Hamamoto, K.; Nagata, S.; Suga, T.J. Structural investigation of the C60/C70 whiskers fabricated by forming liquid-liquid interfaces of toluene with dissolved C60/C70 and isopropyl alcohol. Mater. Res. 2003, 18, 1096–1103. [Google Scholar] [CrossRef]
- Sathish, M.; Miyazawa, K. Size-tunable hexagonal fullerene (C60) nanosheets at the liquid-liquid interface. J. Am. Chem. Soc. 2007, 129, 13816–13817. [Google Scholar] [CrossRef]
- Geng, J.; Zhou, W.; Skelton, P.; Yue, W.; Kinloch, I.A.; Wimdle, A.H.; Johnson, B.F.G. Crystal structure and growth mechanism of unusually long fullerene (C60) nanowires. J. Am. Chem. Soc. 2008, 130, 2527–2534. [Google Scholar]
- Malik, S.; Fujita, N.; Muhopadhyay, P.; Goto, Y.; Kaneko, K.; Ikeda, T.; Shinkai, S. Creation of 1D [60]fullerene superstructures and its polymerization by γ-ray irradiation. J. Mater. Chem. 2007, 17, 2454–2458. [Google Scholar] [CrossRef]
- Miyazawa, K.; Ringor, C. Platinum chloride deposition into C60 nanotubes. Mater. Lett. 2008, 62, 410–413. [Google Scholar] [CrossRef]
- Kato, R.; Miyazawa, K. Cross-sectional structural analysis of C60 nanowhiskers by transmission electron microscopy. Diam. Relat. Mater. 2011, 20, 299–303. [Google Scholar] [CrossRef]
- Holczer, K.; Klein, O.; Griiner, G.; Huang, S.-M.; Kaner, R.B.; Fu, K.-J.; Whetten, R.L.; Diederich, F. Alkali-fulleride superconductors: Synthesis, composition, and diamagnetic shielding. Science 1991, 252, 1154–1157. [Google Scholar]
- Yildirim, T.; Barbedette, L.; Fischer, J.E.; Lin, C.L.; Robert, J.; Petit, P.; Palstra, T.T.M. Tc vs carrier concentration in cubic fulleride superconductors. Phys. Rev. Lett. 1996, 77, 167–170. [Google Scholar] [CrossRef]
- Murphy, D.W.; Rosseinsky, M.J.; Fleming, R.M.; Tycko, R.; Ramirez, A.P.; Haddon, R.C.; Siegrist, T.; Dabbagh, G.; Tully, J.C.; Walstedt, R.E. Synthesis and characterization of alkali metal fullerides: AxC60. J. Phys. Chem. Solids 1992, 53, 1321–1332. [Google Scholar] [CrossRef]
- Kato, R.; Miyazawa, K. Raman laser polymerization of C60 nanowhiskers. J. Nanotechnol. 2012, 2012. [Google Scholar] [CrossRef]
- Watanabe, M.; Hotta, K.; Miyazawa, K.; Tachibana, M. GC-MS analysis of the solvents contained in C60 nanowhiskers. J. Phys.: Conf. Ser. 2009, 159. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Takeya, H.; Miyazawa, K.; Kato, R.; Wakahara, T.; Ozaki, T.; Okazaki, H.; Yamaguchi, T.; Takano, Y. Superconducting Fullerene Nanowhiskers. Molecules 2012, 17, 4851-4859. https://doi.org/10.3390/molecules17054851
Takeya H, Miyazawa K, Kato R, Wakahara T, Ozaki T, Okazaki H, Yamaguchi T, Takano Y. Superconducting Fullerene Nanowhiskers. Molecules. 2012; 17(5):4851-4859. https://doi.org/10.3390/molecules17054851
Chicago/Turabian StyleTakeya, Hiroyuki, Kun’ichi Miyazawa, Ryoei Kato, Takatsugu Wakahara, Toshinori Ozaki, Hiroyuki Okazaki, Takahide Yamaguchi, and Yoshihiko Takano. 2012. "Superconducting Fullerene Nanowhiskers" Molecules 17, no. 5: 4851-4859. https://doi.org/10.3390/molecules17054851



