Synthesis and Characterization of Triphenylphosphine Adducts of Ferrocene-Based Palladacycles and Their Performance in the Suzuki and Sonogashira Reactions with Bromo- and Chloroarenes
Abstract
:1. Introduction

2. Results and Discussion
2.1. Synthesis and Characterization of Complexes 2–3
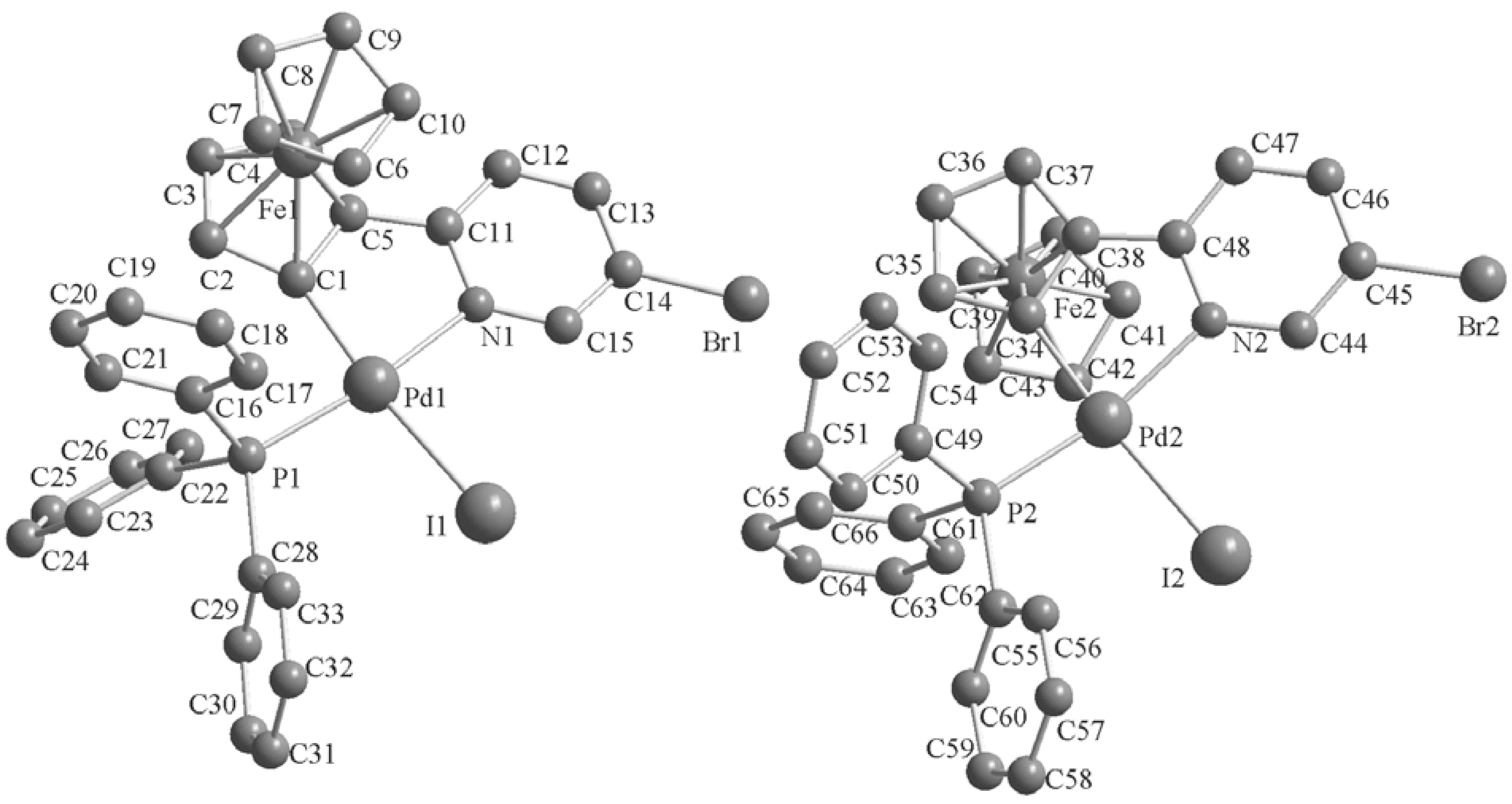
2.2. Crystal Structures of the Complexes 2–3


| Compound | 2a | 3a | 3b |
|---|---|---|---|
| Pd(1)-C[(1) or (5)] | 1.995(2) | 2.003(9) | 1.997(7)[2.011(8)] |
| Pd(1)-N(1) | 2.1418(19) | 2.144(7) | 2.160(6)[2.160(6)] |
| Pd(1)-P(1) | 2.2369(7) | 2.246(2) | 2.238(2)[2.236(2)] |
| Pd(1)-[Cl(2) or I(1)] | 2.3393(8) | 2.6752(12) | 2.6491(10)[2.6574(10)] |
| C[(1) or (5)]-Pd(1)-N(1) | 80.87(9) | 80.3(3) | 81.0(3)[81.1(3)] |
| C[(1) or (5)]-Pd(1)-P(1) | 92.30(8) | 91.8(3) | 90.2(2)[91.0(2)] |
| N(1)-Pd(1)-[Cl(2) or I(1)] | 92.94(6) | 94.79(19) | 93.46(17)[93.23(17)] |
| P(1)-Pd(1)-[Cl(2) or I(1)] | 94.17(3) | 95.20(6) | 95.52(6)[96.40(6)] |


2.3. Application in Suzuki Reaction
| Entry | X | R | Catalyst (mol% Pd) | Yield b (%) |
|---|---|---|---|---|
| 1 | Br | p-CH3 | 2a (0.5) | 93 |
| 2 | Br | p-CH3 | 2b (0.5) | 95 |
| 3 | Br | p-CH3 | 3a (0.5) | 84 |
| 4 | Br | p-CH3 | 3b (0.5) | 82 |
| 5 | Br | o-CH3 | 2a (0.5) | 91 |
| 6 | Br | o-CH3 | 2b (0.5) | 92 |
| 7 | Br | o-CH3 | 3a (0.5) | 73 |
| 8 | Br | o-CH3 | 3b (0.5) | 75 |
| 9 | Br | o-OCH3 | 2a (0.5) | 88 |
| 10 | Br | o-OCH3 | 2b (0.5) | 86 |
| 11 | Br | p-NO2 | 2a (0.1) | 98 |
| 12 | Br | p-NO2 | 2b (0.1) | 97 |
| 13 | Br | p-NO2 | 3a (0.1) | 92 |
| 14 | Br | p-NO2 | 3b (0.1) | 90 |
| 15 | Cl | p-CH3 | 2a (1) | trace |
| 16 | Cl | p-CH3 | 2b (1) | trace |
2.4. Application in Sonogashira Reaction

| Entry | X | R | Catalyst (mol% Pd) | Yield b (%) |
|---|---|---|---|---|
| 1 | Br | H | 2a (1) | 88 |
| 2 | Br | H | 2b (1) | 90 |
| 3 | Br | H | 3a (1) | 96 |
| 4 | Br | H | 3b (1) | 97 |
| 5 | Br | o-CH3 | 2a (1) | 81 |
| 6 | Br | o-CH3 | 2b (1) | 80 |
| 7 | Br | o-CH3 | 3a (1) | 92 |
| 8 | Br | o-CH3 | 3b (1) | 94 |
| 9 | Br | o-CH3 | 3a (1) | 89 |
| 10 | Br | o-CH3 | 3a (1) | 87 |
| 11 | Br | p-COCH3 | 2a (0.1) | 91 |
| 12 | Br | p-COCH3 | 2b (0.1) | 92 |
| 13 | Br | p-COCH3 | 3a (0.1) | 96 |
| 14 | Br | p-COCH3 | 3b (0.1) | 98 |
| 15 | Cl | p-COCH3 | 3a (2) | 35 |
| 16 | Cl | p-COCH3 | 3b (2) | 37 |
| 17 | Cl | p-NO2 | 3a (2) | 46 |
| 18 | Cl | p-NO2 | 3b (2) | 49 |
3. Experimental
3.1. General Procedures
3.2. The Synthesis of Complex 2a
3.3. General Method for the Synthesis of Complexes 3a,b
3.4. General Procedure for the Arylation Reactions
3.5. Crystal Structure Determination
| Compound | 2a | 3a | 3b |
|---|---|---|---|
| Elemental formula | C32H25Cl2FeN2PPd | C32H25ClFeIN2PPd | C33H26BrFeINPPd |
| Formula mass | 701.66 | 793.11 | 836.58 |
| Crystal system | Monoclinic | Monoclinic | Triclinic |
| Space group | P2(1)/c | P2(1)/c | P-1 |
| Crystal size/mm | 0.38 × 0.27 × 0.16 | 0.35 × 0.27 × 0.18 | 0.34 × 0.26 × 0.17 |
| a / Å | 9.2765(7) | 8.835(3) | 10.410(3) |
| b / Å | 15.8968(13) | 16.756(6) | 16.185(4) |
| c / Å | 19.7419(16) | 20.546(7) | 17.981(4) |
| α / o | 90 | 90 | 85.597(3) |
| β / o | 90.8120(10) | 90.789(4) | 86.449(3) |
| γ / o | 90 | 90 | 81.452(3) |
| V / Å3 | 2911.0(4) | 3041.4(18) | 2983.0(12) |
| Dc / g cm−3 | 1.601 | 1.732 | 1.863 |
| Z | 4 | 4 | 4 |
| Data/restraints/parameters | 5419/0/352 | 5623/0/352 | 10997/0/703 |
| R1, wR2 [I > 2σ(I)]a | 0.0251, 0.0551 | 0.0595, 0.1755 | 0.0525, 0.1108 |
| a R1 = Σ||Fo| − |Fc|| / Σ|Fo|, wR2 = [Σ (Fo2 − Fc2)2/ Σw(Fo2)2] 1/2 | |||
4. Conclusions
Acknowledgments
References
- Tsuji, J. Palladium Reagents and Catalysts; Wiley: Chichester, UK, 2004. [Google Scholar]
- Phan, N.T.S.; Sluys, M.V.D.; Jones, C.W. On the nature of the active species in palladium catalyzed Mizoroki-Heck and suzuki-miyaura couplings-homogeneous or heterogeneous catalysis, a critical review. Adv. Synth. Catal. 2006, 348, 609–679. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C. The Sonogashira reactions: A booming methodology in synthetic organic chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef]
- Bedford, R.B. Palladacyclic catalysts in C-C and C-Heteroatom bond-forming reactions. Chem. Commun. 2003, 1787–1796. [Google Scholar] [CrossRef]
- Dupont, J.; Pfeffer, M. Palladacycles; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Alonso, D.A.; Nájera, C. Oxime-derived palladacycles as source of palladium nanoparticles. Chem. Soc. Rev. 2010, 39, 2891–2902. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Z.Q.; Fu, W.J.; Lou, X.H.; Li, Y.F.; Cen, F.F.; Ma, H.J.; Ji, B.M. Synthesis and Structural Characterization of Monophosphine-Cyclopalladated Ferrocenylpyrimidine Complexes and Reusable Catalytic System for Amination of Hindered Aryl Chlorides in PEG-400. Organometallics 2009, 28, 1909–1916. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.P.; Wang, Z.Q.; Fu, W.J.; Hao, X.Q.; Xu, Y.; Ji, B.M. Design and synthesis of tetranuclear cluster monophosphine-cyclopalladated ferrocenylpyrimidinone complexes from the palladium-catalyzed hydroxylation of chloropyrimidine. Chem. Commun. 2010, 46, 6852–6854. [Google Scholar] [CrossRef]
- Xu, C.; Li, H.M.; Liu, H.; Zhang, Z.Q.; Wang, Z.Q.; Fu, W.J.; Zhang, Y.Q. N-Heterocyclic carbene adducts of cyclopalladated ferrocenylpyridine: synthesis, structural characterization and reusable catalytic system for Suzuki and amination of aryl chlorides in poly(ethylene glycol-400) Inorg. Chim. Acta. 2012, 386, 22–26. [Google Scholar]
- Xu, C.; Li, H.M.; Wang, Z.Q.; Fu, W.J.; Zhang, Y.Q.; Ji, B.M. N-heterocyclic carbene adducts of cyclopalladated ferrocenylpyridazine: Synthesis, structural characterization and application in α-arylation of ketones with aryl chlorides. Aust. J. Chem. 2012, 65, 366–370. [Google Scholar]
- Xu, C.; Wang, Z.Q.; Zhang, Y.P.; Dong, X.M.; Hao, X.Q.; Fu, W.J.; Ji, B.M.; Song, M.P. Synthesis and Structural Characterization of Palladacycles with Polydentate Ligands via the Stepwise Coupling Route: Palladacycles Containing Halide as Efficient Catalysts and Substrates. Eur. J. Inorg. Chem. 2011, 4878–4888. [Google Scholar]
- Aakeröy, C.B.; Evans, T.A.; Seddon, K.R.; Pálinkó, I. The C–H···Cl hydrogen bond: Does it exit? New J. Chem. 1999, 23, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Brammer, L.; Bruton, E.A.; Sherwood, P. Understanding the Behavior of Halogens as Hydrogen Bond Acceptors. Cryst Growth Des 2001, 1, 277–290. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F.; Tedesco, E.; Biradha, K.; Desiraju, G.R. Hydrogen Bonding in Organometallic Crystals. 6.† X−H---M Hydrogen Bonds and M---(H−X) Pseudo-Agostic Bonds. Organometallics 1997, 16, 1846–1856. [Google Scholar] [CrossRef]
- Neve, F.; Crispini, A. N,N′-Dodecamethylene-bis(pyridinium) goes lamellar. Role of C–H···I, C–H···M and I···I interactions in the crystal structure of its hexaiododipalladate derivative. CrystEngComm 2003, 5, 265–268. [Google Scholar] [CrossRef]
- Troitskaya, L.L.; Starikova, Z.A.; Demeshchik, T.V.; Ovseenko, S.T.; Vorontsov, E.V.; Sokolov, V.I. Abnormal cyclopalladation of Schiff based made of metallocenyl aldehydes and α-ferrocenylethylamine: Unexpected formation of the heteroannular 3-atomic bridge. J. Organomet. Chem. 2005, 690, 3976–3982. [Google Scholar]
- Xu, C.; Gong, J.F.; Yue, S.F.; Zhu, Y.; Wu, Y.J. Tricyclohexylphosphine-cyclopalladated ferr ocenylimine complexes: synthesis, crystal structures and application in Suzuki and Heck reactions. Dalton. Trans. 2006, 4730–4739. [Google Scholar]
- Bedford, R.B.; Cazin, C.S.J.; Holder, D. The development of palladium catalysts for C-C and C-heteroatom bond forming reactions of aryl chloride substrates. Coord. Chem. Rev. 2004, 248, 2283–2321. [Google Scholar] [CrossRef]
- Farina, V. High-Turnover Palladium Catalysts in Cross-Coupling and Heck Chemistry: A Critical Overview. Adv. Synth. Catal. 2004, 346, 1553–1582. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Böhm, V.P.W.; Reisinger, C.P. Application of palladacycles in Heck type reactions. J. Organomet. Chem. 1999, 576, 23–41. [Google Scholar]
- Bedford, R.B.; Cazin, C.S.J. High-Activity Catalysts for Suzuki Coupling and Amination reactions with Deactivated Aryl Chloride Substrates: Importance of the Palladium Source. Organometallics 2003, 22, 987–999. [Google Scholar] [CrossRef]
- Xu, C.; Gong, J.F.; Guo, T.; Zhang, Y.H.; Wu, Y.J. Cyclopalladated ferrocenylimine complexes with dicyclohexylphosphinobiphenyl ligand ligands: Synthesis, crystal structures and their use highly efficient catalysts for Suzuki reaction of aryl chlorides. J. Mol. Catal. A 2008, 279, 69–76. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C. Recent advances in Sonogashira reactions. Chem. Soc. Rev. 2011, 40, 5084–5121. [Google Scholar] [CrossRef]
- Doucet, H.; Hierso, J.C. Palladium-Based Catalytic Systems for the Synthesis of Conjugated Enynes by Sonogashira Reactions and Related Alkynylations. Angew. Chem. Int. Ed. 2007, 46, 834–871. [Google Scholar] [CrossRef]
- Inés, B.; SanMartin, R.; Churruca, F.; Domínguez, E.; Urtiaga, M.K.; Arriortua, M.I. A nonsymmetric pincer-type palladium catalyst in Suzuki, Sonogashira and Hiyama coupling in neat water. Organometallics 2008, 27, 2833–2839. [Google Scholar] [CrossRef]
- Samantaray, M.K.; Shaikh, M.M.; Ghosh, P. Copper-free and amine-free Sonogashira coupling in air in a mixed aqueous medium by palladium complexes of N/O-functionalized N-heterocyclic carbenes. J. Organomet. Chem. 2009, 694, 3477–3486. [Google Scholar] [CrossRef]
- Alonso, D.A.; Botella, L.; Nájera, C.; Pacheco, M.C. Synthetic applications of oxime-derived palladacycles as versatile catalysts in cross-coupling reactions. Synthesis 2004, 1713–1718. [Google Scholar]
- Consorti, C.S.; Fabricio, R.F.; Rominger, F.; Dupont, J. A simple and efficient copper-free catalytic system based on a palladacycle for the arylation of alkynes. Adv. Synth. Catal. 2006, 348, 133–141. [Google Scholar] [CrossRef]
- Yang, F.; Cui, X.L.; Li, Y.N.; Zhang, J.L.; Ren, G.R.; Wu, Y.J. Cyclopalladated ferrocenylimines: efficient catalysts for homocoupling and Sonogashira reaction of terminal alkynes. Tetrahedron 2007, 63, 1963–1969. [Google Scholar]
- Zhang, B.S.; Wang, C.; Gong, J.F.; Song, M.P. Facile synthesis of achiral and chiral PCN pincer palladium(II) complexes and their application in the Suzuki and copper-free Sonogashira cross-coupling reactions. J. Organomet. Chem. 2009, 694, 2555–2561. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the complexes 1–3 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bai, S.-Z.; Xu, C.; Li, H.-M.; Wang, Z.-Q.; Fu, W.-J. Synthesis and Characterization of Triphenylphosphine Adducts of Ferrocene-Based Palladacycles and Their Performance in the Suzuki and Sonogashira Reactions with Bromo- and Chloroarenes. Molecules 2012, 17, 5532-5543. https://doi.org/10.3390/molecules17055532
Bai S-Z, Xu C, Li H-M, Wang Z-Q, Fu W-J. Synthesis and Characterization of Triphenylphosphine Adducts of Ferrocene-Based Palladacycles and Their Performance in the Suzuki and Sonogashira Reactions with Bromo- and Chloroarenes. Molecules. 2012; 17(5):5532-5543. https://doi.org/10.3390/molecules17055532
Chicago/Turabian StyleBai, Su-Zhen, Chen Xu, Hong-Mei Li, Zhi-Qiang Wang, and Wei-Jun Fu. 2012. "Synthesis and Characterization of Triphenylphosphine Adducts of Ferrocene-Based Palladacycles and Their Performance in the Suzuki and Sonogashira Reactions with Bromo- and Chloroarenes" Molecules 17, no. 5: 5532-5543. https://doi.org/10.3390/molecules17055532