Synthesis of Novel E-2-Chlorovinyltellurium Compounds Based on the Stereospecific Anti-addition of Tellurium Tetrachloride to Acetylene
Abstract
:1. Introduction

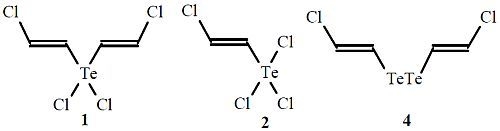
2. Results and Discussion







3. Experimental
3.1. General Information
3.2. Synthetic Procedures for the Preparation of Compounds 1–6
4. Conclusions
Acknowledgements
- Sample Availability: Samples of compound 1 are available from the authors.
References and Notes
- Schwarz, K.; Foltz, C.M. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. J. Am. Chem. Soc. 1957, 79, 3292–3293. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and organotellurium compounds: Toxicology and pharmacology. Chem. Rev. 2004, 104, 6255–6286. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Therapeutic potential of selenium and tellurium compounds: Opportunities yet unrealized. Dalton Trans. 2012, 41, 6390–6395. [Google Scholar] [CrossRef]
- Wieslander, E.; Engman, L.; Svensjö, E.; Erlansson, M.; Johansson, U.; Linden, M.; Andersson, C.M.; Brattsand, R. Antioxidative properties of organotellurium compounds in cell systems. Biochem. Pharmacol. 1998, 55, 573–584. [Google Scholar]
- Garberg, P.; Engman, L.; Tolmachev, V.; Lundqvist, H.; Gerdes, R.G.; Cotgreave, I.A. Binding of tellurium to hepatocellular selenoproteins during incubation with inorganic tellurite: Consequences for the activity of selenium-dependent glutathione peroxidase. Int. J. Biochem. Cell. Biol. 1999, 31, 291–301. [Google Scholar] [CrossRef]
- Sredni, B.; Caspi, R.R.; Klein, A.; Kalechman, Y.; Danziger, Y.; Ya’akov, M.B.; Tamari, T.; Shalit, F.; Albeck, M. A new immunomodulating compound (AS-101) with potential therapeutic application. Nature 1987, 330, 173–176. [Google Scholar]
- Sredni, B.; Xu, R.H.; Albeck, M.; Gafter, U.; Gal, R.; Shani, A.; Tichler, T.; Shapira, J.; Bruderman, I.; Catane, R.; et al. The protective role of the immunomodulator AS101 against chemotherapy-induced alopecia: Studies on human and animal models. Int. J. Cancer 1996, 65, 97–103. [Google Scholar] [CrossRef]
- Sredni-Kenigsbuch, D.; Shohat, M.; Shohat, B.; Ben-Amitai, D.; Chan, C.C.; David, M. The novel tellurium immunomodulator AS101 inhibits interleukin-10 production and p38 MAPK expression in atopic dermatitis. J. Dermatol. Sci. 2008, 50, 232–235. [Google Scholar] [CrossRef]
- Petragnani, N.; Campos, M.M. Organic tellurium compounds. IV. Vinylic and ethynylic tellurium derivatives. Tetrahedron 1962, 18, 527–530. [Google Scholar] [CrossRef]
- Uemura, S.; Miyoshi, H.; Okano, M. Regio- and stereospecific Z-iodo- and Z-bromochlorination of alkylphenylacetylenes via Z-chlorotelluration. Chem. Lett. 1979, 1357–1358. [Google Scholar]
- Chieffi, A.; Menezes, P.H.; Comasseto, J.V. Reduction of organotelluriun trichlorides with sodium borohydryde. Organometallics 1997, 16, 809–811. [Google Scholar]
- Petragnani, N.; Mendes, S.R.; Silveira, C.C. Tellurium tetrachloride: An improved method of preparation. Tetrahedron Lett. 2008, 49, 2371–2372. [Google Scholar]
- Cunha, R.L.O.R.; Zukerman-Schpector, J.; Caracelli, I.; Comasseto, J.V. Revisiting the addition reaction of TeCl4 to alkynes: The crystal structure and docking studies of 1-chloro-2-trichlorotelluro-3-phenyl-propen-2-ol. J. Organometal. Chem. 2006, 691, 4807–4815. [Google Scholar]
- Chauhan, A.K.S.; Bharti, S.N.; Srivastava, R.C.; Butcher, R.J.; Duthie, A. Stereospecific chlorotelluration of terminal acetylenes. J. Organomet. Chem. 2012, 708–709, 75–81. [Google Scholar]
- Zukerman-Schpector, J.; Haiduc, I.; Dabdoub, M.J.; Biazzotto, J.C.; Braga, A.L.; Dornelles, L.; Caracelli, I. Dichloro-bis(2-chloro-2-phenyl-vinyl)Te(IV) and dibromo-bis(2-bromo-2-phenyl-vinyl)Te(IV): Supramolecular self-assembly through different π-aryl interactions. Z. Kristallogr. 2002, 217, 609–614. [Google Scholar] [CrossRef]
- Braverman, S.; Cherkinsky, M.; Jana, R.; Kalendar, Y.; Sprecher, M. Reaction of selenium and telluriun halides with propargyl alcohols. The regio- and stereoselectivity of addition to the triple bond. J. Phys. Org. Chem. 2010, 23, 1114–1120. [Google Scholar]
- Zeni, G.; Ludtke, D.S.; Panatieri, R.B.; Braga, A.L. Vinylic tellurides: From preparation to their applicability in organic synthesis. Chem. Rev. 2006, 106, 1032–1076. [Google Scholar]
- Petragnani, N.; Stefani, H.A. Tellurium in Organic Synthesis; Academic Press: London, UK, 2007. [Google Scholar]
- Petragnani, N.; Stefani, H.A. Advances in organic tellurium chemistry. Tetrahedron 2005, 61, 1613–1679. [Google Scholar] [CrossRef]
- Comasseto, J.V.; Stefani, H.A.; Chiefi, A.; Zukerman-Schpector, J. Addition of organotellurium trihalides to acetylenes. Organometallics 1991, 10, 845–846. [Google Scholar]
- Potapov, V.A.; Amosova, S.V.; Khangurov, A.V.; Petrov, P.A. Synthesis of acetylenic tellurides by the iodomethane-induced reaction of dialkyl ditellurides with phenylacetylene. Phosphorus Sulfur Silicon Relat. Elem. 1993, 79, 273–275. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V. New routes to unsaturated organoselenium and organotellurium compounds. Russ. J. Org. Chem. 1996, 32, 1099–1109. [Google Scholar]
- Potapov, V.A.; Amosova, S.V.; Shestakova, V.Y.; Zhnikin, A.R.; Petrov, B.V. Synthesis of alkyl ethynyl tellurides and 1,2-bis(alkyltelluro) acetylenes by electrophilic-reagent-induced reaction of dialkyl ditellurides with acetylene. Rec. Trav. Chim. 1996, 115, 441–442. [Google Scholar]
- Potapov, V.A.; Amosova, S.V.; Petrov, P.A. Aromatic substitution and dealkylation by alkanetellurolate anions. Tetrahedron Lett. 1992, 33, 6515–6518. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V.; Shestakova, V.Y. Novel synthesis of unsaturated organoselenium and organotellurium compounds based on organic dichalcogenides and elemental chalcogens. Phosphorus Sulfur Silicon Relat. Elem. 1998, 136–138, 205–208. [Google Scholar]
- Potapov, V.A.; Amosova, S.V.; Beletskaya, I.P.; Starkova, A.A.; Hevesi, L. Organic diselenides and ditellurides: Disproportionations, synthesis of stannyl selenides, reactions with acetylenes. Phosphorus Sulfur Silicon Relat. Elem. 1998, 136–138, 591–594. [Google Scholar]
- Potapov, V.A.; Trofimov, B.A. 1-(Organosulfanyl)-, 1-(organoselanyl)-, and 1-(organotellanyl)alk-1-yne. Sci. Synth. 2005, 24, 957–1005. [Google Scholar]
- Potapov, V.A.; Amosova, S.V. New methods for preparation of organoselenium and organotellurium compounds from elemental chalcogens. Russ. J. Org. Chem. 2003, 39, 1373–1380. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Amosova, S.V.; Musalova, M.V.; Penzik, M.V. Reaction of selenium dichloride with divinyl telluride. Russ. J. Org. Chem. 2011, 47, 950–951. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Amosova, S.V.; Musalova, M.V.; Volkova, K.A. Reactions of selenium dichloride and dibromide with diallyl telluride. Russ. J. Gen. Chem. 2011, 81, 2201–2202. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Gusarova, N.K.; Tatarinova, A.A.; Potapov, V.A.; Sinegovskaya, L.M.; Amosova, S.V.; Voronkov, M.G. Alkyl vinyl tellurides from tellurium, acetylene and alkyl halides. Tetrahedron Lett. 1988, 44, 6739–6744. [Google Scholar]
- Potapov, V.A.; Amosova, S.V. Synthesis of vinylic selenides and tellurides by the addition of alkaneselenolate and alkanetellurolate anions to acetylenes. Phosphorus Sulfur Silicon Relat. Elem. 1993, 79, 277–280. [Google Scholar] [CrossRef]
- Gusarova, N.K.; Trofimov, B.A.; Tatarinova, A.A.; Potapov, V.A.; Gusarov, A.V.; Amosova, S.V.; Voronkov, M.G. Reactions of chalcogenes with acetylene .4. Synthesis of divinyl telluride by the direct reaction of tellurium with acetylene. Zh. Org. Khim. 1989, 25, 39–45. [Google Scholar]
- Gusarova, N.K.; Trofimov, B.A.; Tatarinova, A.A.; Potapov, V.A.; Sinegovskaya, L.M.; Amosova, S.V.; Voronkov, M.G. Reactions of chalcogens with acetylene. 3. Alkyl vinyl tellurides from tellurium, acetylene and alkyl halides. Zh. Org. Khim. 1988, 24, 1869–1875. [Google Scholar]
- Trofimov, B.A.; Gusarova, N.K.; Tatarinova, A.A.; Amosova, S.V.; Sinegovskaya, L.M.; Keiko, V.V.; Potapov, V.A. Vinyl methyl telluride. Zh. Org. Khim. 1984, 20, 1802–1802. [Google Scholar]
- Potapov, V.A.; Gusarova, N.K.; Amosova, S.V.; Tatarinova, A.A.; Sinegovskaya, L.M.; Trofimov, B.A. Elementary tellurium reaction with phenylacetylene-Synthesis of 3-benzylidene-4-phenyl-1,2-ditellurole and Z,Z-distyryltelluride. Zh. Org. Khim. 1986, 22, 220–221. [Google Scholar]
- Potapov, V.A.; Amosova, S.V.; Kashik, A.S. Reactions of selenium and tellurium metals with phenylacetylene in 3-phase catalytic systems. Tetrahedron Lett. 1989, 30, 613–618. [Google Scholar] [CrossRef]
- Potapov, V.A.; Kashik, A.S.; Amosova, S.V. Reaction of metal tellurium with phenylacetylene under the phase-transfer catalysis. Zh. Org. Khim. 1988, 24, 2005–2006. [Google Scholar]
- Amosova, S.V.; Martynov, A.V.; Shagun, V.A.; Musalov, M.V.; Larina, L.I.; Krivdin, L.B.; Zhilitskaya, L.V.; Voronkov, M.G. Anti-markovnikov addition of tellurium tetrachloride to trimethyl ethynyl silane. J. Organomet. Chem. 2008, 693, 2509–2513. [Google Scholar]
- Amosova, S.V.; Martynov, A.V.; Penzik, M.V.; Makhaeva, N.A.; Potapov, V.A.; Albanov, A.I.; Zhilitskaya, L.V.; Voronkov, M.G. 4,4-Diorganyl-1,1,3,6-tetrachloro-1,4-tellura(IV)silafulvenes—New class of tellurium-silicon containing heterocycles. J. Organomet. Chem. 2008, 693, 3650–3654. [Google Scholar]
- Trofimov, B.A.; Gusariva, N.K. Acetylene: New prospects of classical reactions. Russ. Chem. Rev. 2007, 76, 507–527. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Musalova, M.V.; Amosova, S.V. Reaction of tellurium tetrachloride with acetylene. Russ. Chem. Bull. 2009, 58, 2404–2405. [Google Scholar] [CrossRef]
- Shmid, G.H. Electrophilic Additions to Carbon-Carbon Triple Bonds. In The Chemistry of the Carbon-Carbon Triple Bond; Patai, S., Ed.; John Wiley & Sons: Chichester, UK, 1978; p. 275. [Google Scholar]
- Dabdoub, M.J.; Dabdoub, V.B.; Comasseto, J.V.; Petragnani, N. Synthesis of vinylic tellurides. J. Organomet. Chem. 1986, 308, 211–222. [Google Scholar]
- Dabdoub, M.J.; Comasseto, J.V. Divinyl ditelluride: Synthesis and reactivity. J. Organomet. Chem. 1988, 344, 167–173. [Google Scholar]
- Amosova, S.V.; Gostevskaya, V.I.; Gavrilova, G.M.; Potapov, V.A.; Kashik, A.S. Preparative synthesis of divinyl ditelluride. Zh. Org. Khim. 1988, 24, 454–455. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Musalova, M.V.; Potapov, V.A.; Amosova, S.V. Synthesis of Novel E-2-Chlorovinyltellurium Compounds Based on the Stereospecific Anti-addition of Tellurium Tetrachloride to Acetylene. Molecules 2012, 17, 5770-5779. https://doi.org/10.3390/molecules17055770
Musalova MV, Potapov VA, Amosova SV. Synthesis of Novel E-2-Chlorovinyltellurium Compounds Based on the Stereospecific Anti-addition of Tellurium Tetrachloride to Acetylene. Molecules. 2012; 17(5):5770-5779. https://doi.org/10.3390/molecules17055770
Chicago/Turabian StyleMusalova, Maria V., Vladimir A. Potapov, and Svetlana V. Amosova. 2012. "Synthesis of Novel E-2-Chlorovinyltellurium Compounds Based on the Stereospecific Anti-addition of Tellurium Tetrachloride to Acetylene" Molecules 17, no. 5: 5770-5779. https://doi.org/10.3390/molecules17055770