Thiadiazines, N,N-Heterocycles of Biological Relevance
Abstract
:1. Introduction

2. Synthesis of Tetrahydro-2H-1,3,5-thiadiazine-2-thione (THTT) Derivatives

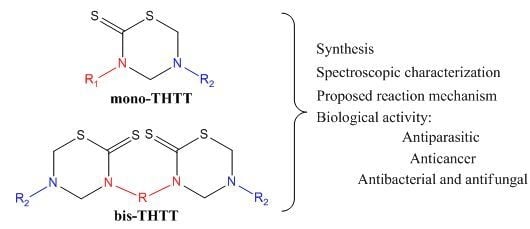
2.1. Synthesis of 3,5-Disubstituted-tetrahydro-2H-1,3,5-thiadiazine-2-thione (Mono-THTT)


2.2. Synthesis of Alkyl-linked-bis-(2-thioxo-[1,3,5] thiadiazinan-3-yl) carboxylic Acids (Bis-THTT)


2.3. Proposed Reaction Mechanism

2.4. Structural Characterization

3. Biological Activity of THTT Derivatives
3.1. Antiparasitic Activity



3.2. Anticancer Activity

3.3. Antibacterial and Antifungal Activity









4. Conclusions
Acknowledgments
References and Notes
- Redtenbacher, J.; Liebig, J. Ueber das Carbothildin. Liebigs Ann. Chem. 1848, 65, 43–45. [Google Scholar] [CrossRef]
- Ainley, A.; Davies, D. The constitution of the so-called carbothialdines and the preparation of some homologous compounds. J. Chem. Soc. 1944, 147–149. [Google Scholar] [CrossRef]
- Vogelsang, H.D.; Wagner-Jauregg, T.; Rebling, R. Homologs of ethylene thiocyanohydrin and their ethers and thio ethers. Justus Liebigs Ann. Chem. 1950, 569, 183–198. [Google Scholar] [CrossRef]
- Cummins, E.W. 3,3'-Hydrocarbonylene bis(tetrahydro-1,3,5-thiadiazine-2-thiones) and fungicidal compositions. US Patent 3085046, 9 April 1963. [Google Scholar]
- Martin, D.; Venker, P. Preparation of S35-labeled carbon disulfide and 2-thio-3-benzyl-5-β-hydroxyethyltetrahydro-1,3,5-thiadiazine. Naturwissenschaften 1962, 49, 256–257. [Google Scholar] [CrossRef]
- Nebioglu, D.; Ertan, R.; Ertan, M. The synthesis and structural analysis of 3,5-disubstituted-tetrahydro-2H-1,3,5-thiadiazine-2-thione derivatives, starting from some biologically active amino acids. J. Fac. Pharm. Istanbul Univ. 1986, 22, 77–86. [Google Scholar]
- Rieche, A.; Hilgetag, G.; Martini, A.; Nejedly, O.; Schlegel, J. New compounds with bactericidal, fungicidal, and virostatic activity. I. 2-Thiotetrahydro-1,3,5-thiadiazine (carbothialdine) and dithiocarbamic acid salts. Arch. Pharm. 1960, 293, 957–967. [Google Scholar] [CrossRef]
- Coro, J.; Piñeiro, R.; Monzote, L.; Rodríguez, H.; Suárez, M. Thiadiazine derivatives as antiprotozoal new drugs. Open Med. Chem. J. 2011, 5, 51–60. [Google Scholar] [CrossRef]
- El-Shorbagi, A.-N. New tetrahydro-2H-1,3,5-thiadiazine-2-thione derivatives as potential antimicrobial agents. Arch. Pharm. Pharm. Med. Chem. 2000, 333, 281–286. [Google Scholar] [CrossRef]
- Aboul-Fadl, T.; Hussein, M.A.; El-Shorbagi, A.-N.; Khallil, A.-R. New 2H-tetrahydro-1,3,5-thiadiazine-2-thiones incorporating glycine and glycinamide as potential antifungal agents. Arch. Pharm. Pharm. Med. Chem. 2002, 9, 438–442. [Google Scholar]
- Vitangelo, M.; Vovlas, N. Herbicides and nematocides for celery seedbeds. Inf. Fitopatol. 1975, 25, 17–21. [Google Scholar]
- Zsolnai, T. Antimicrobial activity of potential isothiocyanate formers. Arzneimittelforschung 1968, 18, 1319–1324. [Google Scholar]
- Katiyar, D.; Tiwari, V.K.; Tripathi, R.P.; Srivastava, A.; Chaturvedi, V.; Srivastava, R.; Srivastava, B.S. Síntesis and antimycobacterial activity of 3,5-disubstituted thiadiazine thiones. Bioorgan. Med. Chem. 2003, 11, 4369–4375. [Google Scholar] [CrossRef]
- El-Shorbagi, A.-N. Model for delivery of amines through incorporation into a tetrahydro-2H-1,3,5-thiadiazine-2-thione structure. Eur. J. Med. Chem. 1994, 29, 11–15. [Google Scholar] [CrossRef]
- Sasaki, T.; Fujikawa, Y.; Sakota, R.; Sakashita, M.; Hibi, M. 3,5-Bis(phenylmethyl)tetrahydro-2H-1,3,5-thiadiazine-2-thione for arteriosclerosis treatment. JPN. Kokai Tokkyo Koho, JP 62036319 A, 17 February 1987. [Google Scholar]
- Semreen, M.H.; El-Shorbagi, A.-N.; Al-Tel, T.H.; Alsalahat, I.M.M. Targeting γ-aminobutyric acid (GABA) carriers to the brain: Potential relevance as antiepileptic pro-drugs. Med. Chem. 2010, 6, 144–149. [Google Scholar]
- Aboul-Fadl, T.; El-Shorbagi, A.-N. New prodrug approach for amino acids and amino-acid-like drugs. Eur. J. Med. Chem. 1996, 31, 165–169. [Google Scholar] [CrossRef]
- Abd-Elrahman, M.I.; Ahmed, M.O.; Ahmed, S.M.; Aboul-Fadl, T.; El-Shorbagi, A. Kinetics of solid state stability of glycine derivatives as a model for peptides using differential scanning calorimetry. Biophys. Chem. 2002, 97, 113–120. [Google Scholar] [CrossRef]
- El-Shorbagi, A.-N. Disubstituted tetrahydro-2H-1,3,5-thiadiazine-2-thiones as lipophilic carriers for glutamine and glutamic acid. Bull. Pharm. Sci. 2000, 23, 31–38. [Google Scholar]
- Chen, G.; He, J.; Zhang, F.; Li, Y.; Li, Y. Synthesis and structure-activity relationship of new kind inhibitor of mercapto-proteinases. Shoudu Yike Daxue Xuebao 2002, 23, 107–109. [Google Scholar]
- El-Shorbagi, A.-N. New tetrahydro-2H-1,3,5-thiadiazine-2-thione derivatives as potential antimicrobial agents. Arch. Pharm. 2000, 333, 281–286. [Google Scholar] [CrossRef]
- Aboul-Fadl, T.; Hassanin, K. Tetrahydro-2-H-1,3,5-thiadiazine-5-(4-pyridylcarboxamide)-2-thione derivatives as prodrugs for isoniazid: Synthesis, investigations and in vitro antituberculous activity. Pharmazie 1999, 54, 244–247. [Google Scholar]
- Ochoa, C.; Pérez, R.; Pérez, E.; Suárez, M.; Ochoa, E.; Rodríguez, H.; Gómez, B.; Muelas, S.; Nogal, J.J.; Martínez, R.A. Synthesis and Antiprotozoan properties of new 3,5-Disubstituted-Tetrahydro-2H-1,3,5-Thiadiazin-2-Thione derivatives. Arzneimittel-Forschung 1999, 49, 764–769. [Google Scholar]
- Pérez, R.; Suárez, M.; Rodríguez, H.; Ochoa, C. Study on the Decomposition Products Thiadiazinthione and their Anti-Cancer Properties. Arzneimittel-Forschung 2000, 50, 854–857. [Google Scholar]
- Aboul-Fadl, T.; Khallil, A.R. Synthesis, degradation kinetics and in vitro antimicrobial activity of tetrahydro-2H-1,3,5-thiadiazine-2-thione derivatives of some beta-amino acids. Arzneimittel-Forschung 2003, 53, 526–531. [Google Scholar]
- Ozcelik, A.B.; Ersan, S.; Ural, A.U.; Ozkan, S.; Ertan, M. Synthesis of 3-substituted-5-(4-carboxycyclohexylmethyl)-tetrahydro-2H-1,3,5-thiadiazine-2-thione derivatives as antifibrinolytic and antimicrobial agents. Arzneimittel-Forschung 2007, 57, 554–559. [Google Scholar]
- Hussein, M.A.; El-Shorbagi, A.N.; Khallil, A.R. Synthesis and antifungal activity of 3,3'-ethylenebis (5-alkyl-1,3,5-thiadiazine-2-thiones). Arch. Pharm. Pharm. Med. Chem. 2001, 334, 305–308. [Google Scholar] [CrossRef]
- El Bialy, S.A.A.; Abdelal, A.M.; El-Shorbagi, A.N.; Kheira, S.M.M. 2,3-Bis(5-alkyl-2-thiono-1,3,5-thiadiazin-3-yl) propionic acid: One-pot Domino synthesis and antimicrobial activity. Arch. Pharm. Chem. Life Sci. 2005, 338, 38–43. [Google Scholar] [CrossRef]
- Coro, J.; Pérez, R.; Rodríguez, H.; Suárez, M.; Vega, C.; Rolon, M.; Montero, D.; Nogal, J.J.; Gómez-Barrio, A. Synthesis and antiprotozoan evaluation of new alkyl-linked bis(2-thioxo-[1,3,5]thiadiazinan-3-yl) carboxylic acids. Bioorg. Med. Chem. 2005, 13, 3413–3421. [Google Scholar]
- Coro, J.; Little, S.; Yardley, V.; Suarez, M.; Rodríguez, H.; Martín, N.; Perez-Pineiro, R. Synthesis and Antiprotozoal evaluation of New N4-(benzyl)Spermidyl-linked bis(1,3,5-thiadiazinane-2-thione). Arch. Pharm. 2008, 341, 318–329. [Google Scholar]
- Saglam, E.; Sarac, S.; Kilic, E.; Ozalp, M.; Ertan, M. Synthesis and antimicrobial activity of some 3,5-disubstituted-tetrahydro-2H-1,3,5-thiadiazine-2-thione derivatives. Turk. J. Pharm. Sci. 2011, 8, 159–168. [Google Scholar]
- Pérez, R.; Reyes, O.; Suárez, M.; Garay, H.E.; Cruz, L.J.; Rodríguez, H.; Molero-Vichez, M.D.; Ochoa, C. Solid phase synthesis of 3-(5'-carboxypentyl)-5-substituted tetrahydro-2H-1,3,5-thiadizin-2-thione derivatives. Tetrahedron Lett. 2000, 41, 613–616. [Google Scholar]
- O’Sullivan, M.C.; Zhou, Q.; Li, Z.; Durham, T.B.; Rattendi, D.; Lane, S.; Bacchi, C.J. Polyamine derivatives as inhibitors of Trypanothione reductase and assessment of their tripanocidal activities. Bioorgan. Med. Chem. 1997, 5, 2145–2155. [Google Scholar] [CrossRef]
- Coro, J.; Álvarez, R.; Montero, A.L.; Suárez, M.; Martin, N.; Perez-Pineiro, R. A computational approach to the synthesis of 1,3,5-thiadiazinane-2 thiones in aqueous medium: Theoretical evidence for water-promoted heterocyclization. J. Mol. Model. 2008, 14, 641–647. [Google Scholar] [CrossRef]
- Pérez, R.; Suárez, M.; Rodríguez, H.; Ochoa, C. Study on the Decomposition Products Thiadiazinthione and their Anti-Cancer Properties. Arzneimittel-Forschung 2000, 50, 854–857. [Google Scholar]
- Pérez, R.; Suárez, M.; Rodríguez, H.; Molero, D.; Martin, N.; Martínez, R.; Seoane, C. 1H and 13C Spectra Assignment of 3,5-Disubstituted Tetrahydro-2H-1,3,5-Thiadiazin-2-Thione Derivatives. Magn. Reson. Chem. 2001, 39, 22–24. [Google Scholar]
- Pérez, R.; Rodríguez, H.; Suárez, M.; Martín, N.; Seoane, C.; Novoa, H.; Blaton, N.; Peeters, O.; De Ranter, C. A joint theoretical and experimental structural study of N,N-disubstituted tetrahydro-2H-1,3,5-thiadiazines. Tetrahedron 2001, 57, 9543–9549. [Google Scholar]
- Martínez, R.; Martín, N.; Seoane, C.; Suarez, M.; Perez, R.; Rodriguez, H.; Kayali, N. Study of the Electrospray Ionization and Ion Trapp Fragmentation of negative ions of new 3,5-disubstituted tretahydro-(2H)-1,3,5-thiadizine-2-thione. Rapid Commun. Mass Spectrom. 2001, 15, 758–762. [Google Scholar]
- Molero, D.; Coro, J.; Pérez, R.; Suárez, M.; Martínez, R.; Herrera, A.; Martín, N. 1H and 13C-NMR spectral Assignment of Alkyl and Polyamine-linked Bis (2-thioxo-[1,3,5]thiadiazinan-3-yl) carboxylic acids. Magn. Reson. Chem. 2007, 45, 93–98. [Google Scholar]
- North, M.J.; Mottran, J.C.; Coombs, G.H.M.J. Cysteine proteinases of parasitic protozoa. Parasitol. Today 1990, 6, 270–275. [Google Scholar] [CrossRef]
- Goksoyr, J. Chemical and fungicidal reactions of 3,5-dimethyl-tetrahydro-l,3,5-thiadiazine-2-thione. Acta Chem. Scand. 1964, 18, 1341–1352. [Google Scholar] [CrossRef]
- Muelas, S.; Suárez, M.; Pérez, R.; Rodríguez, H.; Ochoa, C.; Escario, J.A.; Gómez-Barrio, A. In vitro and in vivo assays of 3,5,-disubstituted-tetrahydro-2H-1,3,5-thiadiazin-2-thione derivatives against Tripanosoma cruzi. Mem. Inst. Oswaldo Cruz 2002, 97, 269–272. [Google Scholar]
- Monzote, L.; Montalvo, A.M.; Fonseca, L.; Pérez, R.; Suárez, M.; Rodríguez, H. Effect of thiadiazine derivatives on intracellular amastigotes of Leishmania amazonensis. Mem. Inst. Oswaldo Cruz 2004, 99, 329–330. [Google Scholar] [CrossRef]
- Monzote, L.; Montalvo, A.M.; Fonseca, L.; Pérez, R.; Suárez, M.; Rodríguez, H. In vitro activities of thiadiazine derivatives against Leishmania amazonensis. Arzneimittel-Forschung 2005, 55, 232–238. [Google Scholar]
- Coro, J.; Atherton, R.; Little, S.; Wharton, H.; Yardeley, V.; Alvarez, A.; Suárez, M.; Pérez, R.; Rodríguez, H. Alkyl-linked bis-THTT derivatives as potent in vitro trypanocidal agents. Bioorg. Med. Chem. Lett. 2006, 16, 1312–1315. [Google Scholar] [CrossRef]
- Carrasco, R.; Padrón, J.A.; Pérez, R.; Rodríguez, H.; Suárez, M.; Ochoa, C. Quantitative Structure Antitumoral-Activity Relationships of Thiadiazinthione Derivatives Using the Novel Hybrid Molecular Index. J. Pharm. Pharm. Sci. 2005, 8, 586–592. [Google Scholar]
- Radwan, A.A.; Al-Dhfyan, A.; Abdel-Hamid, M.K.; Al-Badr, A.A.; Aboul-Fadl, T. 3,5-Disubstituted thiadiazine-2-thiones: New cell-cycle inhibitors. Arch. Pharm. Res. 2012, 35, 35–49. [Google Scholar] [CrossRef]
- Hussein, A.H.; Hashem, M. Synthesis of new 3-substituted-5-(2-hydroxyethyl)-3,4,5,6-tetrahydro-2H-1,3,5-thiadiazine-2-thione derivatives with potential antimicrobial activity. Arch. Pharm. Chem. Life Sci. 2008, 341, 370–376. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rodríguez, H.; Suárez, M.; Albericio, F. Thiadiazines, N,N-Heterocycles of Biological Relevance. Molecules 2012, 17, 7612-7628. https://doi.org/10.3390/molecules17077612
Rodríguez H, Suárez M, Albericio F. Thiadiazines, N,N-Heterocycles of Biological Relevance. Molecules. 2012; 17(7):7612-7628. https://doi.org/10.3390/molecules17077612
Chicago/Turabian StyleRodríguez, Hortensia, Margarita Suárez, and Fernando Albericio. 2012. "Thiadiazines, N,N-Heterocycles of Biological Relevance" Molecules 17, no. 7: 7612-7628. https://doi.org/10.3390/molecules17077612