Synthesis, Structure and Insecticidal Activities of Some Novel Amides Containing N-Pyridylpyrazole Moeities
Abstract
:1. Introduction

2. Results and Discussion
2.1. Chemistry

| Compd. | R1 | R2 | R3 | R4 | R5 | Compd. | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 7a | H | Cl | H | H | H | 7k | CH3 | H | H | H | NO2 |
| 7b | H | H | F | H | H | 7l | Cl | H | NO2 | H | H |
| 7c | H | H | Cl | H | H | 7m | Br | H | NO2 | H | H |
| 7d | H | H | I | H | H | 7n | NO2 | H | Cl | H | H |
| 7e | H | H | NO2 | H | H | 7o | Cl | H | H | Cl | H |
| 7f | H | H | OC2H5 | H | H | 7p | CH3 | H | Cl | H | CH3 |
| 7g | H | Cl | F | H | H | 7q | CH3 | H | Br | H | CH3 |
| 7h | CH3 | H | CH3 | H | H | 7r | CH3 | H | NO2 | H | Cl |
| 7i | CH3 | H | NO2 | H | H | 7s | CH3 | H | Cl | H | NO2 |
| 7j | CH3 | H | H | H | CH3 | 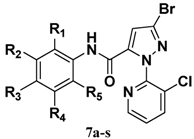 |
2.2. Crystal Structure


2.3. Insecticidal Activities and Structure-Activity Relationship (SAR)
| Compd. | Mythimna separata Walker | Culex pipiens pallens | |||||
|---|---|---|---|---|---|---|---|
| µg·mL−1/death rate (%) | µg·mL−1/death rate (%) | ||||||
| 200 | 100 | 50 | 25 | 10 | 5 | 2 | |
| 7a | 0 | — a | |||||
| 7b | 100 | 100 | 100 | 50 | 0 | ||
| 7c | 100 | 100 | 100 | 70 | 0 | 10 | |
| 7d | 100 | 100 | 50 | 100 | |||
| 7e | 100 | 90 | 20 | 20 | |||
| 7f | 100 | 0 | 30 | ||||
| 7g | 100 | 0 | — | ||||
| 7h | 100 | 70 | 0 | — | |||
| 7i | 100 | 100 | 100 | 80 | 50 | 40 | |
| 7j | 100 | 80 | 40 | — | |||
| 7k | 100 | 80 | 20 | 50 | |||
| 7l | 100 | 100 | 100 | 100 | 80 | 0 | 80 |
| 7m | 100 | 100 | 30 | 40 | |||
| 7n | 10 | 40 | |||||
| 7o | 0 | 10 | |||||
| 7p | 100 | 100 | 100 | 80 | 20 | 30 | |
| 7q | 100 | 100 | 100 | 100 | 30 | 30 | |
| 7r | 100 | 100 | 100 | 70 | 30 | — | |
| 7s | 100 | 100 | 100 | 60 | 20 | ||
| RynaxypyrTM | 100 | 100 | |||||

| Compd. | Plutella xylostella (Linnaeus, 1758) | Laphygma exigua Hübner | ||||||
|---|---|---|---|---|---|---|---|---|
| µg·mL−1/death rate (%) | µg·mL−1/death rate (%) | |||||||
| 200 | 100 | 50 | 25 | 200 | 100 | 50 | 25 | |
| 7b | 100 | 98 | 99 | 88 | 95 | 100 | 92 | 96 |
| 7c | 100 | 98 | 100 | 97 | 100 | 100 | 100 | 100 |
| 7m | 100 | 98 | 96 | 90 | 100 | 100 | 100 | 100 |
| 7l | 78 | 72 | 0 | 91 | 86 | 78 | 65 | |
| 7p | 100 | 100 | 97 | 94 | 100 | 100 | 98 | 94 |
| 7q | 89 | 72 | 0 | — a | 100 | 100 | 89 | 93 |
| 7s | 95 | 89 | 84 | 76 | 98 | 100 | 95 | 92 |
| RynaxypyrTM | — | — | 100 | 94 | — | — | 100 | 100 |
| Ck | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
3. Experimental
3.1. General
3.2. Chemical Synthesis
3.2.1. 3-Chloro-2-hydrazinylpyridine (2)
3.2.2. Ethyl 2-(3-Chloro-2-pyridinyl)-5-oxopyrazolidine-3-carboxylate (3)
3.2.3. Ethyl 3-Bromo-1-(3-chloro-2-pyridinyl)-4,5-dihydro-1H-pyrazole-5-carboxylate (4)
3.2.4. Ethyl 3-Bromo-1-(3-chloro-2-pyridinyl)-1H-pyrazole-5-carboxylate (5)
3.2.5. 3-Bromo-1-(3-chloro-2-pyridinyl)-1H-pyrazole-5-carboxylic acid (6)
3.2.6. General Procedure for the Synthesis of Compounds 7a–s
3.3. Crystal Structure Determination

3.4. Biological Assay
3.4.1. Stomach Toxicity against M. separata Walker
3.4.2. Toxicity against Culex pipiens pallens
3.4.3. Stomach Toxicity against Plutella xylostella (Linnaeus, 1758) and Laphygma exigua Hübner
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Nauen, R. Insecticide mode of action: Return of the ryanodine receptor. Pest Manag. Sci. 2006, 62, 690–692. [Google Scholar] [CrossRef]
- Lahm, G.P.; Pasteris, R.J.; Stevenson, T.M. Pyrazole and pyrrole carboxamide insecticides. WO Patent 2,003,106,427, 24 December 2003. Chem. Abstr. 2003, 140, 42172. [Google Scholar]
- Ebbinghaus-Kintscher, U.; Luemmena, P.; Lobitz, N.; Schulte, T.; Funke, C.; Fischer, R.; Masaki, T.; Yasokawa, N.; Tohnishi, M. Phthalic acid diamides activate ryanodine-sensitiveCa2+ release channels in insects. Cell Calcium 2006, 39, 21–33. [Google Scholar] [CrossRef]
- Cordova, D.; Benner, E.A.; Sacher, M.D.; Rauh, J.J.; Sopa, J.S.; Lahm, G.P.; Selby, T.P.; Stevenson, T.M.; Flexner, L.; Gutteridge, S.; et al. Anthranilic diamides: A new class of insecticides with a novel mode of action, Ryanodine receptor activation. Pest. Biochem. Physiol. 2006, 84, 196–214. [Google Scholar] [CrossRef]
- Lahm, G.P.; Selby, T.P.; Freudenberger, J.H.; Stevenson, T.M.; Myers, B.J.; Seburyamo, G.S.; Smith, B.K.; Flex-ner, L.; Clark, C.E.; Cordova, D. Insecticidal anthranilic diamides: A new class of potent ryanodine receptor activators. Bioorg. Med. Chem. Lett. 2005, 15, 4898–4906. [Google Scholar]
- Lahm, G.P.; Stevenson, T.M.; Selby, T.P.; Freudenberger, J.H.; Cordova, D.; Flexner, L.; Bellin, C.A.; Dubas, C.M.; Smith, B.K.; Hughes, K.A.; et al. Rynaxypyr (TM): A new insecticidal anthranilic diamide that acts as a potent and selective ryanodine receptor activator. Bioorg. Med. Chem. Lett. 2007, 17, 6274–6279. [Google Scholar]
- Dong, W.L.; Xu, J.Y.; Liu, X.H.; Li, Z.M.; Li, B.J.; Shi, Y.X. Synthesis, Crystal structure and biological activity of novel anthranilic diamides containing 1,2,3-thiadiazole. Chem. J. Chin. Univ. 2008, 29, 1990–1994. [Google Scholar]
- Dong, W.L.; Liu, X.H.; Xu, J.Y.; Li, Z.M. Design and synthesis of novel anthranilic diamides containing 5,7-dimethyl[1,2,4]triazolo[1,5-a]pyrimidine. J. Chem. Res. 2008, 530–533. [Google Scholar]
- Dong, W.L.; Xu, J.Y.; Liu, X.H.; Xiong, L.X.; Li, Z.M. Synthesis, Structure and biological activities of some novel anthranilic acid esters containing N-Pyridylpyrazole. Chin. J. Chem. 2009, 27, 579–586. [Google Scholar] [CrossRef]
- Xu, J.Y.; Dong, W.L.; Xiong, L.X.; Li, Y.X.; Li, Z.M. Design, Synthesis and biological activities of novel amides (Sulfonamides) containing N-Pyridylpyrazole. Chin. J. Chem. 2009, 27, 2007–2012. [Google Scholar] [CrossRef]
- Shapiro, R.; Taylor, E.G.; Zimmerman, W.T. Method for preparing N-phenylpyrazole-1-carboxamides. WO Patent 2,006,062,978, 15 June 2006. Chem. Abstr. 2006, 145, 62887. [Google Scholar]
- Lahm, G.P.; Selby, T.P.; Stevenson, T.M. Arthropodicidal anthranilamides. WO Patent 2,003,015,519, 27 February 2003. Chem. Abstr. 2003, 138, 200332. [Google Scholar]
- Liu, X.H.; Pan, L.; Tan, C.X.; Weng, J.Q.; Wang, B.L.; Li, Z.M. Synthesis, Crystal structure, Bioactivity and DFT calculation of new oxime ester derivatives containing cyclopropane moiety. Pestic. Biochem. Physiol. 2011, 101, 143–147. [Google Scholar] [CrossRef]
- Xue, Y.L.; Zhang, Y.G.; Liu, X.H. Synthesis, Crystal structure and biological activity of 1-Cyano-N-(4-bromophenyl)cyclopropanecarboxamide. Asian J. Chem. 2012, 24, 3016–3018. [Google Scholar]
- Liu, X.H.; Pan, L.; Weng, J.Q.; Tan, C.X.; Li, Y.H.; Wang, B.L.; Li, Z.M. Synthesis, Structure, and biological activity of novel (oxdi/tri)azoles derivatives containing 1,2,3-thiadiazole or methyl moiety. Mol. Divers. 2012, 16, 251–260. [Google Scholar] [CrossRef]
- Tan, C.X.; Weng, J.Q.; Liu, Z.X.; Liu, X.H.; Zhao, W.G. Synthesis, Crystal structure, and Fungicidal activity of a novel 1,2,3-Thidiazole compound. Phosphorus Sulfur Silicon Relat. Elem. 2012, 187, 990–996. [Google Scholar] [CrossRef]
- Liu, X.H.; Tan, C.X.; Weng, J.Q.; Liu, H.J. (E)-(4-Bromobenzylidene)amino cyclopropanecarboxylate. Acta Cryst. 2012, 68, o493. [Google Scholar]
- Liu, X.H.; Tan, C.X.; Weng, J.Q. Synthesis, Dimeric crystal structure, and Fungicidal activity of 1-(4-Methylphenyl)-2-(5-((3,5-Dimethyl-1H-Pyrazol-1-yl)methyl)-4-Phenyl-4H-1,2,4-Trizol-3-ylthio)Ethanone. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 558–564. [Google Scholar] [CrossRef]
- Liu, X.F.; Liu, X.H. 5-(4-Pyridyl)-1,3,4-thiadiazole-2(3H)-thione. Acta Cryst. 2011, 67, o202. [Google Scholar]
- Chen, P.Q.; Tan, C.X.; Weng, J.Q.; Liu, X.H. Synthesis, Structure and DFT calculation of chlorimuron-ethyl. Asian J. Chem. 2012, 24, 2808–2810. [Google Scholar]
- Xue, Y.L.; Liu, X.H.; Zhang, Y.G. Synthesis, Crystal structure and biological activity of 1-Cyano-N-phenylcyclopropanecarboxamide. Asian J. Chem. 2012, 24, 1571–1574. [Google Scholar]
- Liu, H.J.; Weng, J.Q.; Tan, C.X.; Liu, X.H. 1-Cyano-N-(2,4,5-trichlorophenyl)cyclopropane-1-carboxamide. Acta Cryst. 2011, 67, o1940. [Google Scholar]
- Xue, Y.L.; Zhang, Y.G.; Liu, X.H. Synthesis, Crystal structure and biological activity of 1-Cyano-N-(2,4-dichlorophenyl)cyclopropanecarboxamide. Asian J. Chem. 2012, 24, 5087–5089. [Google Scholar]
- Liu, X.H.; Pan, L.; Ma, Y.; Weng, J.Q.; Tan, C.X.; Li, Y.H.; Shi, Y.X.; Li, B.J.; Li, Z.M.; Zhang, Y.G. Design, Synthesis, Biological activities, and 3D-QSAR of new N,N'-Diacylhydrazines containing 2-(2,4-dichlorophenoxy)propane Moiety. Chem. Biol. Drug Des. 2011, 78, 689–694. [Google Scholar] [CrossRef]
- Clark, D.A.; Lahm, G.P.; Smith, B.K.; Barry, J.D.; Clagg, D.G. Synthesis of insecticidal fluorinated anthranilic diamides. Bioorg. Med. Chem. 2008, 16, 3163–3170. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS97 and SHELXL97; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Wilson, A.J. International Table for X-ray Crystallography; Kluwer Academic Publisher: Dordrecht, The Netherlands, 1992; Volume C, pp. 219–222. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar]
- Zhao, Q.Q.; Li, Y.Q.; Xiong, L.X.; Wang, Q.M. Design, Synthesis and insecticidal activity of novel phenylpyrazoles containing a 2,2,2-Trichloro-1-alkoxyethyl moiety. J. Agric. Food Chem. 2010, 58, 4992–4998. [Google Scholar] [CrossRef]
- Raymond, M.; Marquine, M. Evolution of insecticide resistance in Culex pipiens polulations: The Corsican paradox. J. Evol. Biol. 1994, 7, 315–337. [Google Scholar]
- Sun, R.F.; Zhang, Y.L.; Chen, L.; Li, Y.Q.; Li, Q.S.; Song, H.B.; Huang, R.Q.; Bi, F.C.; Wang, Q.M. Design, Synthesis and insecticidal activities of new N-Benzoyl-N′-phenyl-N′-sulfenylureas. J. Agric. Food Chem. 2009, 57, 3661–3668. [Google Scholar] [CrossRef]
- Sayyed, A.H.; Ferre, J.; Wright, D.J. Mode of inheritance and stability of resistance to Bacillus thuringiensis var kurstaki in a diamondback moth (Plutella xylostella) population from Malaysia. Pest Manage. Sci. 2000, 56, 743–748. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dong, W.-L.; Xu, J.-Y.; Xiong, L.-X.; Li, Z.-M. Synthesis, Structure and Insecticidal Activities of Some Novel Amides Containing N-Pyridylpyrazole Moeities. Molecules 2012, 17, 10414-10428. https://doi.org/10.3390/molecules170910414
Dong W-L, Xu J-Y, Xiong L-X, Li Z-M. Synthesis, Structure and Insecticidal Activities of Some Novel Amides Containing N-Pyridylpyrazole Moeities. Molecules. 2012; 17(9):10414-10428. https://doi.org/10.3390/molecules170910414
Chicago/Turabian StyleDong, Wei-Li, Jing-Ying Xu, Li-Xia Xiong, and Zheng-Ming Li. 2012. "Synthesis, Structure and Insecticidal Activities of Some Novel Amides Containing N-Pyridylpyrazole Moeities" Molecules 17, no. 9: 10414-10428. https://doi.org/10.3390/molecules170910414




