Structural Characterization and Identification of Major Constituents in Jitai Tablets by High-Performance Liquid Chromatography/Diode-Array Detection Coupled with Electrospray Ionization Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion


| Peak No. | tR (min) | λmax (nm) | M.W | MS and MSn fragments (%) | Name | Origin | |
|---|---|---|---|---|---|---|---|
| MS + ( m/z) | MS − ( m/z) | ||||||
| 1 | 11.7 | 230, 406 | 485.3 | MS2[486]: 436(100), 404(44) | Mesaconine | 1 | |
| MS3[486→436]: 404(100), 372(22), 293(21) | |||||||
| 2 | 13.4 | 228, 260, 294 | 154.1 | MS2[153]: 109(100) | Procatechuic acid | 2 | |
| 3 | 14.8 | 280, 288 | 198.1 | MS2[395]: 197(100), 179(2) | Danshensu a | 2 | |
| 4 | 15.5 | 276, 339 | 788.7 | MS2[787]: 625(100), 301(2), 463(3) | 6-Hydroxy kaempferol (3 × Glc/Gal) | 3 | |
| 5 | 16.0 | 266, 347 | 802.2 | MS2[801]: 625(100), 668(3), 463(3) | 6-Hydroxy kaempferol (2 × Glc + GluA) | 3 | |
| MS3[801→625]: 463(100), 301(31) | |||||||
| 6 | 16.8 | 303.1 | MS2[304]: 156(78), 138(100), 110(22) | Scopolamine a | 12 | ||
| 7 | 17.7 | 256, 352 | 640.5 | MS2[639]: 463(100), 301(6) | Quercetin (Glc/Gal + GluA) | 3 | |
| MS3[639→463]: 301(100), 271(10) | |||||||
| 8 | 18.0 | 295, 324 | 180.1 | MS2[180]: 163(100) | MS2[179]: 135(100) | Caffeic acid a | 2 |
| 9 | 18.4 | 225, 403 | 612.2 | MS2[611]: 491(100), 403(28), 473(7) | Hydroxysafflor yellow A a | 3 | |
| 10 | 18.8 | 457.1 | MS2[502]: 456(100), 323(8), | Amygdalin a | 11 | ||
| MS3[502→456]: 323(100), 221(11), 179(8) | |||||||
| 11 | 19.1 | 256, 350 | 626.5 | MS2[625]: 463(100), 557(11), 577(10), 301(9) | Quercetin 2 × Glc/Gal | 3 | |
| MS3[625→463]: 301(100), 254(2) | |||||||
| 12 | 19.8 | 280, 340 | 934.8 | MS2[933]: 771(100), 625(21) MS3[933→771]: 609(100), 301(7), 463(5) | 6-Hydroxy kaempferol (2 × Glc/Gal + Rut) | 3 | |
| 13 | 19.5 | 266, 340 | 640.1 | MS2[639]: 463(100), 571(38), MS3[639→463]: 301(100), 256(3) | 6-Hydroxy kaempferol (Glc/Gal + GluA) | 3 | |
| 14 | 21.0 | 276, 339 | 624.1 | MS2[623]: 447(100), MS3[623→477]: 285(100) | Kaempferol (Glc/Gal + GluA) | 3 | |
| 15 | 22.6 | 289.1 | MS2[290]: 221(100), 124(30) MS3[290→221]: 203(100) | Atropine | 12 | ||
| 16 | 23.5 | 295.2 | MS2[340]: 294(100), 161(22) | Prunasin | 11 | ||
| 17 | 24.0 | 271, 335 | 758.2 | MS2[757]: 595(100), 287(9) MS3[757→595]: 287(100), 329(5) | Carthamidin (Glc/Gal + Rut) | 3 | |
| 18 | 24.4 | 252, 332 | 334.3 | MS2[335]: 317(100), 299(18) MS3[335→317]: 299(100) | MS2[333]: 315(100), 297(15) MS3[333→315]: 297(100) | 5,6,7,8-Tetrahydroxy-tetrahydro-2-[2-(4'-hydroxyphenyl)-ethyl]chromone | 6 |
| 19 | 24.7 | 265, 347 | 772.2 | MS2[771]: 609(100), 463(15) MS3[771→609]: 301(100) | 6-Hydroxykaempferol (Glc/Gal + Rut) | 3 | |
| 20 | 25.0 | 265, 347 | 626.5 | MS2[625]: 463(100), 301(33) MS3[625→463]: 301(100) | 6-Hydroxykaempferol (2 × Glc/Gal) | 3 | |
| 21 | 28.1 | 271, 335 | 758.2 | MS2[757]: 449(100), 287(28) MS3[757→449]: 287(100) | Carthamidin (Glc/Gal + Rut) | 3 | |
| 22 | 28.6 | 253, 336 | 318.1 | MS2[319]: 301(100), 283(22), 255(7) MS3[319→301]: 287(100) | MS2[363]: 317(100), 299(25), 281(6) MS3[363→317]: 281(100) | Isoagarotetrol | 6 |
| 23 | 30.1 | 238, 280, 322 | 194.1 | MS2[177]: 145(100) | MS2[193]: 178(100) | Ferulic acid a | 2 |
| 24 | 30.4 | 280 | 341.1 | MS2[342]: 178(100), 163(21) MS3[342→178]: 163 | Tetrahydrocolumbamine | 10 | |
| 25 | 31.7 | 253, 339 | 318.1 | MS2[319]: 301(100), 283(25), 255(7) MS3[319→301]: 283(100) | MS2[363]: 317(100), 299(23), 281(6) MS3[363→317]: 281(100) | Agarotetrol | 6 |
| 26 | 32.4 | 256, 353 | 593.2 | MS2[592]: 472(100), 364(21), 446(14) MS3[592→472]: 244(100), 364(42) | Hydroxyl cartormin | 3 | |
| 27 | 32.5 | 280 | 355.1 | MS2[356]: 192(100), 165(13) | Tetrahydropulmatine a | 10 | |
| MS3[356→192]: 177(100) | |||||||
| 28 | 34.1 | 285 | 353.1 | MS2[354]: 188(100), 149(93), 159(8) | Protopine a | 10 | |
| MS3[354→188]: 159(100) | |||||||
| 29 | 34.3 | 270, 335 | 450.1 | MS2[449]: 287(100), 259(5) | Carthamidin/isocarthamidin (Glc/Gal) | 3 | |
| MS3[449→287]: 259(100) | |||||||
| 30 | 34.9 | 256, 353 | 592.2 | MS2[593]: 285(100), 257(22) | Kaempferol-3-O-Rutinoside | 3 | |
| MS3[593→285]: 257(100) | |||||||
| 31 | 35.4 | 230, 403 | 1044.3 | MS2[1043]: 1026(100), 863(7) | Anhydrosafflor yellow B | 3 | |
| MS3[1043→1026]: 863(100) | |||||||
| 32 | 36.5 | 254, 288 | 538.1 | MS2[537]: 339(100), 295(17) | Salvianolic acid I/H | 2 | |
| MS3[537→339]: 295(100) | |||||||
| 33 | 36.8 | 280 | 355.7 | MS2[356]: 325(100), 294(7) | Glaucine | 10 | |
| MS3[356→325]: 294(100) | |||||||
| 34 | 36.9 | 280 | 338.3 | MS2[338]: 323(100), 294(8) | Columbamine | 10 | |
| MS3[338→323]: 294(100) | |||||||
| 35 | 37.1 | 238, 330 | 340.1 | MS2[339]: 295(100), 277(8) | Salvianolic acid G | 2 | |
| MS3[339→295]: 277(100) | |||||||
| 36 | 37.2 | 248, 328 | 418.3 | MS2[417]: 209(100), 194(22) | Salvianolic acid D | 2 | |
| MS3[417→209]: 194(100) | |||||||
| 37 | 37.6 | 234, 288 | 716.3 | MS2[715]: 393(100), 257(5) | Dedihydro-salvianolic acid B/isomer | 2 | |
| MS3[715→393]: 257(100) | |||||||
| 38 | 37.7 | 277 | 320.3 | MS2[320]: 292(100), 302(50) | Coptisine | 10 | |
| MS3[320→292]: 292(100), 275(75) | |||||||
| 39 | 38.1 | 240 | 589.3 | MS2[590]: 540(100), 508(5) | Benzoylmesaconitine | 1 | |
| MS3[590→540]: 508(100) | |||||||
| 40 | 38.5 | 270, 340 | 575.2 | MS2[574]: 454(100), 304(15) | Cartormin | 3 | |
| MS3[574→454]: 304(100) | |||||||
| 41 | 38.6 | 288 | 323.1 | MS2[324]: 149(100), 176(5) | Tetrahydrocoptisine | 10 | |
| MS3[324→149]: 176(100) | |||||||
| 42 | 39.7 | 275 | 224.2 | MS2[207]: 165(100), 135(7) | Senkyunolide I | 6/7 | |
| MS3[207→165]: 135(100) | |||||||
| 43 | 39.7 | 238, 330 | 718.1 | MS2[717]: 519(100), 321(6) | Salvianolic acid E | 2 | |
| MS3[717→519]: 321(100) | |||||||
| 44 | 40.0 | 285 | 340.1 | MS2[340]: 176(100), 149(16) | Tetrahydroberineper | 10 | |
| MS3[340→176]: 119(100) | |||||||
| 45 | 40.3 | 224, 328 | 360.1 | MS2[359]: 161(100), 133(12) | Rosmarimic acid | 2 | |
| MS3[359→161]: 133(100) | |||||||
| 46 | 40.4 | 280 | 369.4 | MS2[370]: 165(89),192(100) | Corydaline | 10 | |
| MS3[370→192]: 149(100), 177(85) | |||||||
| 47 | 40.5 | 232, 254, 310 | 538.1 | MS2[556]: 341(100), 295(5) | MS2[537]: 493(100), 295(19) | Lithospermic acid | 2 |
| MS3[556→341]: 295(100) | MS3[537→493]: 295(100) | ||||||
| 48 | 41.0 | 243 | 603.3 | MS2[604]: 586(100), 554(33), 522(7) | Benzoylaconine | 1 | |
| MS3[604→586]: 554(100), 522(33) | |||||||
| 49 | 41.3 | 275 | 224.2 | MS2[207]: 189(100), 145(15) | Senkyunolide H | 6/7 | |
| MS3[207→189]: 145(100) | |||||||
| 50 | 41.7 | 933.1 | MS2[932]: 799(8), 637(100), 475(3) | Notoginsenoside R1 | 4 | ||
| MS3[932→637]: 475(100) | |||||||
| 51 | 41.7 | 232, 288 | 494.1 | MS2[493]: 295(100), 159(17) | Salvianolic acid A | 2 | |
| MS3[493→295]: 159(100) | |||||||
| 52 | 42.6 | 220, 235, 280 | 750.2 | MS2[749]: 339(100), 321(8) | 8-Hydroxy-9''-methyl-, salvianolate B | 2 | |
| MS3[749→339]: 321(100) | |||||||
| 53 | 42.9 | 275 | 352.1 | MS2[352]: 337(100), 308(32) | Palmatine | 10 | |
| MS3[352→337]: 308(100) | |||||||
| 54 | 43.2 | 228, 403 | 614.2 | MS2[613]: 551(100), 533(15) | Safflomin C | 3 | |
| MS3[613→551]: 533 | |||||||
| 55 | 43.4 | 235, 405 | 573.3 | MS2[574]: 542(100), 510(15) | Benzoylhypoaconine | 1 | |
| MS3[574→542]: 510(100) | |||||||
| 56 | 43.6 | 801.1 | MS2[846]: 800(100), 637(23), 475(18) | Ginsenoside Rg1a | 4 | ||
| MS3[846→800]: 637(100), 475(5) | |||||||
| 57 | 43.8 | 277 | 336.3 | MS2[366]: 321(100), 292(5) | Berberine | 10 | |
| MS3[366→321]: 292 | |||||||
| 58 | 43.9 | 947.1 | MS2[946]: 637(100), 475(5) | Ginsenoside Re a | 4 | ||
| MS3[946→637]: 475(100) | |||||||
| 59 | 44.6 | 220, 235, 280 | 750.2 | MS2[749]: 551(100), 321(18) | 8-Hydroxy-9'''-methyl-, salvianolate B | 2 | |
| MS3[749→551]: 321(100) | |||||||
| 60 | 44.7 | 216, 234, 288 | 718.1 | MS2[717]: 519(100), 321(6) | Salvianolic acid B a | 2 | |
| MS3[717→519]: 321(100) | |||||||
| 61 | 46.0 | 265 | 366.1 | MS2[366]: 351(100), 322(22) | Dehydrocorydaline | 10 | |
| MS3[366→351]: 322(100) | |||||||
| 62 | 49.4 | 225, 240, 280 | 732.2 | MS2[731]: 533(100), 335(21) | 4-Methoxyl-salvianolic acid B | 2 | |
| MS3[731→533]: 335(100) | |||||||
| 63 | 50.1 | 1241.4 | MS2[1240]: 1107(100), 945(22), 783(7) | Ginsenoside Ra3/notoginsenoside F | 4 | ||
| MS3[1240→1107]: 945(100), 783(25) | |||||||
| 64 | 52.2 | 224, 288, 324 | 492.1 | MS2[491]: 293(100), 265(33), 249(20) | Salvianolic acid C | 2 | |
| MS3[491→293]: 265(100), 249(12) | |||||||
| 65 | 52.3 | 230, 280, 425 | 294.5 | MS2[295]: 177(100), 145(5) | MS2[293]: 193(100), 179(42) | 6-Gingerol | 5 |
| MS3[295→177]: 145(100) | MS3[293→193]: 179(100) | ||||||
| 66 | 56.7 | 801.1 | MS2[800]: 637(100), 475(25) | Pseudoginsenoside-F11 a | 4 | ||
| MS3[800→637]: 475(100) | |||||||
| 67 | 57.2 | 1211.3 | MS2[1210]: 1078(100), 945(13) | Ginsenoside Fc/Ra1/Ra2 | 4 | ||
| MS3[1210→1078]: 945(100) | |||||||
| 68 | 57.4 | 1109.3 | MS2[1108]: 946(100), 784(7) | Ginsenoside Rb1 a | 4 | ||
| MS3[1108→946]: 784(100) | |||||||
| 69 | 57.8 | 770.9 | MS2[815]: 770(100), 637(12), 475(5) | Notoginsenoside R2 | 4 | ||
| MS3[815→770]: 637(100), 475(26) | |||||||
| 70 | 58.0 | 1151.3 | MS2[1150]: 1108(100), 946(6) | Quinquenoside R1 | 4 | ||
| MS3[1150→1108]: 946(100) | |||||||
| 71 | 58.2 | 1079.3 | MS2[1078]: 945(100), 783(22), 621(31), 459(7) | Ginsenoside Rb2 a | 4 | ||
| MS3[1078→945]: 783(100) | |||||||
| 72 | 58.5 | 1079.2 | MS2[1078]: 945(100), 783(56), 621(19), 459(4) | Ginsenoside Rb3 a | 4 | ||
| MS3[1078→945]: 783(100), 621(21) | |||||||
| 73 | 58.7 | 1121.3 | MS2[1120]: 1078(100), 945(12) | Ginsenoside Rs1/Rs2 | 4 | ||
| MS3[1120→1078]: 945(100) | |||||||
| 74 | 59.0 | 638.4 | MS2[683]: 637(100) | Ginsenoside F1 a | 4 | ||
| 75 | 59.2 | 1121.3 | MS2[1120]: 1078(100), 945(44) | Ginsenoside Rs1/Rs2 | 4 | ||
| MS3[1120→1078]: 945(100) | |||||||
| 76 | 59.4 | 957.1 | MS2[956]: 794(100), 613(6), MS3[956→794]: 613(100) | Ginsenoside Ro | 4 | ||
| 77 | 60.0 | 947.1 | MS2[946]: 784(100), 621(45), 459(7) | Ginsenoside Rd a | 4 | ||
| 78 | 60.7 | 1033.3 | MS2[1032]:, 988(16), 946(100), 784(22), 621(4) | Malonyl ginsenoside Rd | 4 | ||
| MS3[1032→946]: 784(100) | |||||||
| 79 | 63.2 | 280 | 192.1 | MS2[193]: 147(100), 175(22) | Senkyunolide A | 6/7 | |
| MS3[193→147]: 105(100) | |||||||
| 80 | 63.7 | 242, 349 | 296.3 | MS2[297]: 206(100), 140(24) | MS2[295]: 204(100), 138(22) | 6-Hydroxy-7-methoxy-2-[(2-phenyl)-ethyl]chromone | 6 |
| MS3[297→206]: 140(100) | MS3[295→204]: 138(100) | ||||||
| 81 | 64.0 | 785.2 | MS2[784]: 621(100), 475(32) | Ginsenoside Rg3 a | 4 | ||
| MS3[784→621]: 475(100) | |||||||
| 82 | 64.5 | 225, 277, 425 | 308.6 | MS2[291]: 191(100), 176(34) | Methyl-[6]-gingerol | 5 | |
| MS3[291→191]: 176(100) | |||||||
| 83 | 65.1 | 240, 260, 310 | 188.1 | MS2[189]: 171(100), 143(5) | E-butenyl phthalide | 6/7 | |
| MS3[189→171]: 143(100) | |||||||
| 84 | 65.6 | 227, 330 | 266.1 | MS2[267]: 176(100), 110(12) | MS2[265]: 174(100), 146(17) | 6-Hydroxy-2-[(2 phenyl)-ethyl] chromone a | 6 |
| MS3[267→176]: 110(100) | MS3[265→174]: 146(100) | ||||||
| 85 | 67.8 | 230, 280, 425 | 322.3 | MS2[305]: 177(100), 145(7) | MS2[321]: 193(100), 178(6) | 8-Gingerol | 5 |
| MS3[305→177]: 145(100) | |||||||
| 86 | 68.9 | 328, 240 | 310.1 | MS2[311]: 220(100), 205(45) | MS2[309]: 218(100), 203(13) | 6, 7-Dimethoxy-2-[(2-phenyl)-ethyl]chromone | 6 |
| MS3[311→220]: 205(100) | MS3[309→218]: 203(100) | ||||||
| 87 | 71.2 | 206, 280, 326 | 190.2 | MS2[191]: 173(100), 145(22) | Z-ligustilide | 6/7 | |
| MS3[191→173]: 145(100) | |||||||
| 88 | 72.1 | 225 | 232.1 | MS2[233]: 215(100), 187(14) | Costunolide a | 8 | |
| MS3[233→215]: 187(100) | |||||||
| 89 | 72.9 | 225, 275, 420 | 276.2 | MS2[277]: 137(100), 122(5) | 6-Shogaol | 5 | |
| MS3[277→137]: 122(100) | |||||||
| 90 | 73.5 | 230 | 230.1 | MS2[231]: 213(100), 185(42) | Dihydrocostunolide a | 8 | |
| MS3[231→213]: 185(100) | |||||||
| 91 | 74.1 | 230, 275, 422 | 338.3 | MS2[321]: 163(100), 131(24) | 3- or 5-Acetoxy-[6]-gingerdiol | 5 | |
| MS3[321→163]: 131(100) | |||||||
| 92 | 74.5 | 230, 275, 422 | 380.2 | MS2[398]: 261(100), 163(13) | Diacetoxy-[6]-gingerdiol | 5 | |
| MS3[398→261]: 163(100) | |||||||
| 93 | 75.1 | 218, 264, 360 | 296.1 | MS2[297]: 253(100), 211(11) | Cryptotanshinone a | 2 | |
| MS3[297→253]: 211(100) | |||||||
| 94 | 76.4 | 250, 270,354 | 294.1 | MS2[295]: 277(100), 249(19) | Isotanshinone IIA | 2 | |
| MS3[295→277]: 249(100) | |||||||
| 95 | 77.2 | 230, 282, 430 | 350.4 | MS2[333]: 177(100) | MS2[349]: 193(100), 178(15) | 10-Gingerol | 5 |
| MS3[349→193]: 178(100) | |||||||
| 96 | 78.4 | 280 | 380.1 | MS2[381]: 191(100), 173(24) | Tokinolide B | 6/7 | |
| MS3[381→191]:173(100), 155(21) | |||||||
| 97 | 79.0 | 232, 280, 425 | 394.2 | MS2[412]: 275(100), 177(44) | Methyl diacetoxy -[6]-gingerdiol | 5 | |
| MS3[412→275]: 177(100) | |||||||
| 98 | 81.1 | 280 | 380.1 | MS2[381]: 191(100), 173(11), | Riligustilide | 6/7 | |
| MS3[381→191]: 173(100), 155(42) | |||||||
| 99 | 81.6 | 280 | 380.1 | MS2[381]: 191(100), 173(51), | Levistolide A | 6/7 | |
| MS3[381→191]: 173(100), 155(12) | |||||||
| 100 | 82.6 | 270, 354 | 294.1 | MS2[295]: 277(100), 249(14) | Tanshinone IIA a | 2 | |
| MS3[295→277]:249(100) | |||||||
| 101 | 87.0 | 225, 275, 420 | 332.3 | MS2[333]: 137(100), 122(23) | 10-Shogaol | 5 | |
| MS3[333→137]: 122(100) | |||||||
2.1. Identification of Alkaloids in JTT

2.2. Identification of Phenolic Acids in JTT
2.3. Identification of Tanshinones in JTT
2.4. Identification of Flavonoid Glycosides in JTT
2.5. Identification of Cyanogenic Glycosides in JTT

2.6. Identification of Ginsenosides in JTT
2.7. Identification of 2-(2-Phenylethyl) Chromones in JTT
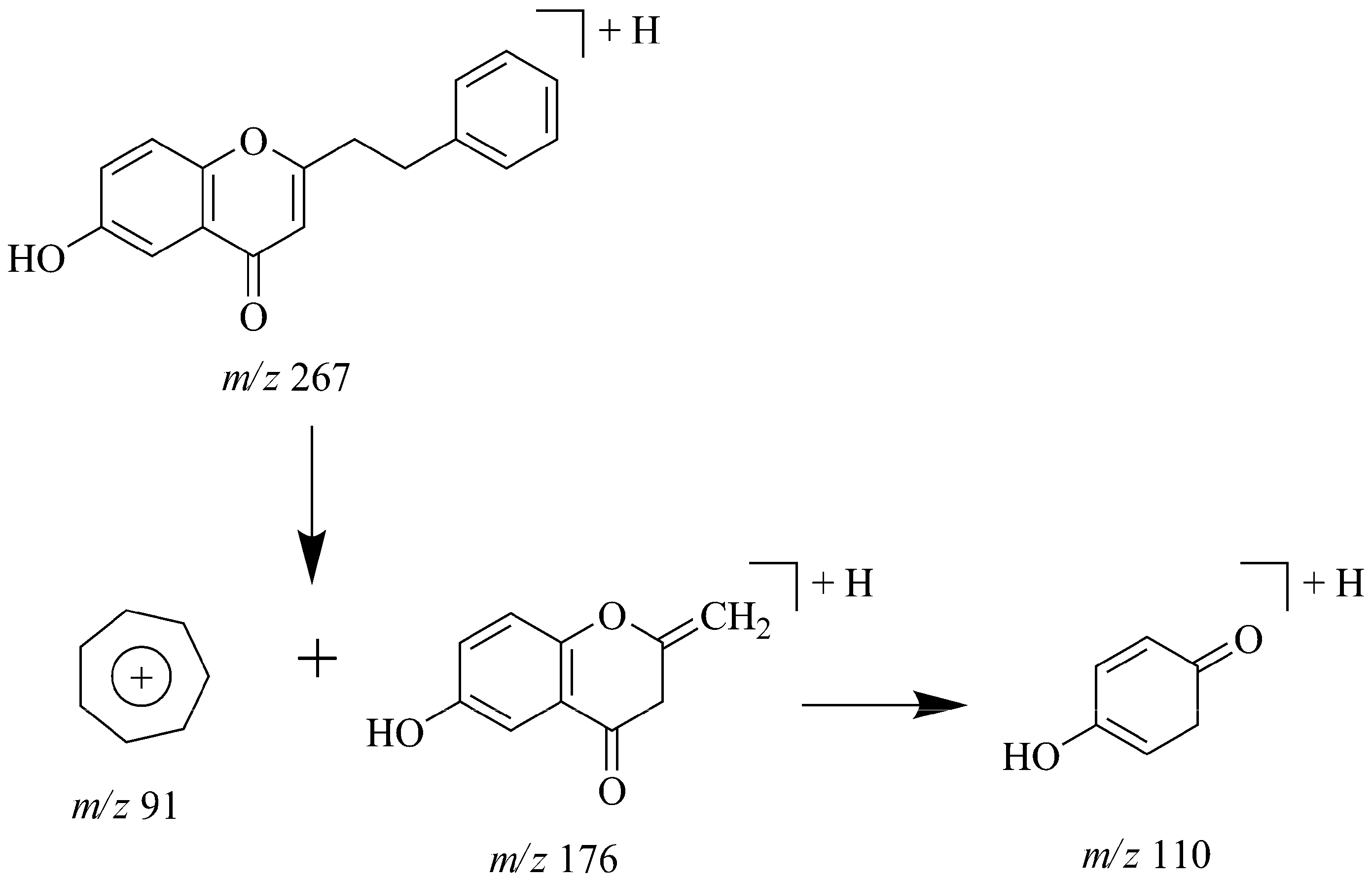
2.8. Identification of Phthalides in JTT
2.9. Identification of Gingerol-Related Compounds in JTT
3 Experimental
3.1. Chemicals and Reagents
3.2. Sample Preparation
3.2.1. Preparation of Analytical Sample of JTT
3.2.2. Preparation of Standard Solutions
3.3. HPLC-DAD/ESI-MS/MS
3.4. Data Analysis
4. Conclusions
Acknowledgements
Conflicts of Interest
References
- Li, L.J.; Xing, X.F.; Shao, H.X. Preparation of traditional Chinese herbs on addiction detoxification: recent progress. Chin. Med. 2003, 34, 20–22. [Google Scholar]
- Shi, J.; Liu, Y.L.; Fang, Y.X.; Xu, G.Z.; Zhai, H.F.; Lu, L. Traditional Chinese medicine in treatment of opiate addiction. Acta Pharmacol. Sin. 2006, 27, 1303–1308. [Google Scholar] [CrossRef]
- Xiong, J.G.; Xiao, Z.X.; Li, J.; Qin, D.S.; Min, M.S.; Yang, J.H.; Wang, Y.H.; Yan, L.; Shu, Q.L. Clinical effect of Jitai tablet combined with lofexidine in the treatment of heroin dependence. Chin. J. Drug Depend. 2001, 4, 290–292. [Google Scholar]
- Tu, Q.X.; Zhao, H.G.; Cheng, Y.P.; Chen, Y.M.; Huang, X.P.; Chen, Y.M.; Han, M. Comparision study on clinical efficacy of Jitai capsule with lofexidine in the treatment of opiate addicts. Chin. J. Drug Depend. 1999, 4, 285–287. [Google Scholar]
- Zhang, H.Y.; Hu, P.; Luo, G.A.; Liang, Q.L.; Wang, Y.L.; Yan, S.K.; Wang, Y.M. Screening and identification of multi-component in Qingkailing injection using combination of liquid chromatography/time-of-flight mass spectrometry and liquid chromatography/diode array detection/ion trap mass spectrometry. Anal. Chim. Acta 2006, 577, 190–200. [Google Scholar] [CrossRef]
- Xu, S.J.; Yang, L.; Zeng, X.; Zhang, M.; Wang, Z.T. Characterization of compounds in the Chinese herbal drug Mu-Dan-Pi by liquid chromatography coupled to electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 3275–3288. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, G.R.; Zhang, Q.Y.; Wu, H.L.; Wu, Y.T. Fingerprint analysis of the fruits of Cnidium monnieri extract by high-performance liquid chromatography-diode array detection-electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2007, 43, 926–936. [Google Scholar] [CrossRef]
- Chen, L.L.; Qi, J.; Chang, Y.X.; Zhu, D.N.; Yu, B.Y. Identification and determination of the major constituents in traditional Chinese medicinal formula Danggui-Shaoyao-San by HPLC-DAD-ESI-MS/MS. J. Pharm. Biomed. Anal. 2009, 50, 127–137. [Google Scholar] [CrossRef]
- Quirantes-Piné, R.; Funes, L.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. High-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight and ion-trap tandem mass spectrometry to identify phenolic compounds from a lemon verbena extract. J. Chromatogr. A 1216, 5391–5397. [Google Scholar]
- Lou, Z.Y.; Zhang, H.; Gong, C.G.; Zhu, Z.Y.; Zhao, L.; Xu, Y.J.; Wang, B.; Zhang, G.Q. Analysis of lignans in Schisandra chinensis and rat plasma by high performance liquid chromatography diode array detection, time of flight mass spectrometry and quadrupole ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 831–842. [Google Scholar] [CrossRef]
- García-Reyes, J.F.; Hernando, M.D.; Ferrer, C.; Molina-Díaz, A.; Fernández-Alba, A.R. Large scale pesticide multiresidue methods in food combining liquid chromatography-time-of-flight mass spectrometry and tandem mass spectrometry. Anal. Chem. 2007, 79, 7308–7323. [Google Scholar]
- Ferrer, I.; Heine, C.E.; Thurman, E.M. Combination of LC/TOF-MS and LC/ion trap MS/MS for the identification of diphenhydramine in sediment samples. Anal. Chem. 2004, 76, 1437–1444. [Google Scholar] [CrossRef]
- Dugo, P.; Cacciola, F.; Donato, P.; Jacques, R.A.; Caramão, E.B.; Mondello, L. High efficiency liquid chromatography techniques coupled to mass spectrometry for the characterization of mate extracts. J. Chromatogr. A 2009, 1216, 7213–7221. [Google Scholar]
- Chuang, W.C.; Young, D.S.; Liu, L.K.; Sheu, S.J. Liquid chromatographic-electrospray mass spectrometric analysis of Coptidis Rhizoma. J. Chromatogr. A 1996, 755, 19–26. [Google Scholar] [CrossRef]
- Cheng, Z.; Guo, Y.; Wang, H.; Chen, G. Qualitative and quantitative analysis of quaternary ammonium alkaloids from Rhizoma Corydalis by matrix-assisted laser desorption/ionization Fourier transform mass spectrometry coupled with a selective precipitation reaction using Reinecke salt. Anal. Chim. Acta 2006, 555, 269–277. [Google Scholar] [CrossRef]
- Zhang, H.G.; Sun, Y.; Duan, M.Y.; Chen, Y.J.; Zhong, D.F.; Zhang, H.Q. Separation and identification of Aconitum alkaloids and their metabolites in human urine. Toxicon 2005, 46, 500–506. [Google Scholar] [CrossRef]
- Yue, H.; Pi, Z.; Song, F.; Liu, Z. Studies on the aconitine-type alkaloids in the roots of Aconitum Carmichaeli Debx. by HPLC/ESIMS/MSn. Talanta 2009, 77, 1800–1807. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Y.; Wang, H.; Du, P. Analysis of scopolamine and its eighteen metabolites in rat urine by liquid chromatography-tandem mass spectrometry. Talanta 2005, 67, 984–991. [Google Scholar] [CrossRef]
- Liu, A.H.; Guo, H.; Ye, M.; Lin, Y.H.; Sun, J.H.; Guo, D.A. Detection, characterization and identification of phenolic acids in Danshen using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. J. Chromatogr. A 1161, 170–182. [Google Scholar]
- Shen, Y.; Wang, X.; Xu, L.; Liu, X.; Chao, R. Characterization of metabolites in rat plasma after intravenous administration of salvianolic acid A by liquid chromatography/time of flight mass spectrometry and liquid chromatography/diode array detection/ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 1810–1816. [Google Scholar] [CrossRef]
- Liu, A.H.; Lin, Y.H.; Yang, M.; Guo, H.; Guan, H.S.; Sun, J.H.; Guo, D.A. Development of the fingerprints for the quality of the roots of Salvia miltiorrhiza and its related preparations by HPLC-DAD and LC-MSn. J. Chromatogr. B 2007, 846, 32–41. [Google Scholar] [CrossRef]
- Li, P.; Wang, G.J.; Li, J.; Hao, H.P.; Zheng, C.N. Characterization of metabolites of tanshinone IIA in rats by liquid chromatography/tandem mass spectrometry. J. Mass Spectrom. 2006, 41, 670–684. [Google Scholar] [CrossRef]
- Jin, Y.; Xiao, Y.; Zhang, F.; Xue, X.; Xu, Q.; Liang, X.M. Systematic screening and characterization of flavonoid glycosides in Carthamus tinctorius L. by liquid chromatography/UV diode-array detection/electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2008, 46, 418–430. [Google Scholar] [CrossRef]
- Wen, A.D.; Yang, J.; Jia, Y.Y.; Yang, Z.F.; Tian, Y.; Wu, Y.; Wang, Z.R.; He, Z.G. A rapid and sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for the determination of hydroxysafflor yellow A in human plasma: Application to a pharmacokinetic study. J. Chromatogr. B 2008, 876, 41–46. [Google Scholar] [CrossRef]
- Ge, B.; Chen, H.; Han, F.; Chen, Y. Identification of amygdalin and its major metabolites in rat urine by LC-MS/MS. J. Chromatogr. B 2007, 857, 281–286. [Google Scholar] [CrossRef]
- Liu, S.; Cui, M.; Liu, Z.; Song, F.; Mo, W. Structural analysis of saponins from medicinal herbs using electrospray ionization tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2004, 15, 133–141. [Google Scholar] [CrossRef]
- Woo, S.S.; Song, J.S.; Lee, J.Y.; In, D.S.; Chung, H.J.; Liu, J.R.; Choi, D.W. Selection of high ginsenoside producing ginseng hairy root lines using targeted metabolic analysis. Phytochemistry 2004, 65, 2751–2761. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Cai, Z. Chemical investigation on Sijunzi decoction and its two major herbs Panax ginseng and Glycyrrhiza uralensis by LC/MS/MS. J. Pharm. Biomed. Anal. 2006, 41, 1642–1647. [Google Scholar] [CrossRef]
- Yagura, T.; Ito, M.; Kiuchi, F.; Honda, G.; Shimada, Y. Four new 2-(2-phenylethyl) chromone derivatives from withered wood of Aquilaria sinensis. Chem. Pharm. Bull. 2003, 51, 560–564. [Google Scholar] [CrossRef]
- Alkhathlan, H.; Al-Hazimi, H.; Al-Dhalaan, F.; Mousa, A. Three 2-(2-phenylethyl) chromones and two terpenes from agarwood. Nat. Prod. Res. 2005, 19, 367–372. [Google Scholar] [CrossRef]
- Yi, T.; Leung, K.S.Y.; Lu, G.H.; Chan, K.; Zhang, H. Simultaneous qualitative and quantitative analyses of the major constituents in the rhizome of Ligusticum Chuanxiong using HPLC-DAD-MS. Chem. Pharm. Bull. 2006, 54, 255–259. [Google Scholar] [CrossRef]
- Zschocke, S.; Klaiber, I.; Bauer, R.; Vogler, B. HPLC-coupled spectroscopic techniques (UV, MS, NMR) for the structure elucidation of phthalides in Ligusticum chuanxiong. Mol. Divers. 2005, 9, 33–39. [Google Scholar] [CrossRef]
- Yang, F.; Xiao, Y.S.; Zhang, F.F.; Xue, X.Y.; Xu, Q.; Liang, X.M. High performance liquid chromatography-mass spectrometry analysis of radix Angelica sciensis. Acta Pharmacol. Sin. 2006, 41, 1078–1081. [Google Scholar]
- Jiang, H.L.; Somogyi, A.; Timmermann, B.N.; Gang, D.R. Instrument dependence of electrospray ionization and tandem mass spectrometric fragmentation of the gingerols. Rapid Commun. Mass Spectrom. 2006, 20, 3089–3096. [Google Scholar] [CrossRef]
- Huang, H.Y.; Kuo, K.L.; Hsieh, Y.Z. Determination of cinnamaldehyde, cinnamic acid, paeoniflorin, glycyrrhizin and [6]-gingerol in the traditional Chinese medicinal preparation Kuei-chih-tang by cyclodextrin-modified micellar electrokinetic chromatography. J. Chromatogr. A 1997, 771, 267–274. [Google Scholar] [CrossRef]
- Jiang, H.L.; Xie, Z.; Koo, H.J.; McLaughlin, S.P.; Timmermann, B.N.; David, R.G. Metabolic profiling and phylogenetic analysis of medicinal Zingiber species: Tools for authentication of ginger (Zingiber officinale Rosc.). Phytochemistry 2006, 67, 1673–1685. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the danshensu, scopolamine, caffeic acid, amygdalin, hydroxysafflor yellow A, ferulic acid, tetrahydropulmatine, salvianolic acid B, pseudoginsenoside-F11, protopine, costunolide, dihydrocostunolide, cryptotanshinone, tanshinone IIA, ginsenoside Rg1, Re, Rb1, Rb2, Rb3, F1, Rd and Rg3 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, S.; Liu, L.; Wang, L.; Hu, Y.; Zhang, W.; Liu, R. Structural Characterization and Identification of Major Constituents in Jitai Tablets by High-Performance Liquid Chromatography/Diode-Array Detection Coupled with Electrospray Ionization Tandem Mass Spectrometry. Molecules 2012, 17, 10470-10493. https://doi.org/10.3390/molecules170910470
Wang S, Liu L, Wang L, Hu Y, Zhang W, Liu R. Structural Characterization and Identification of Major Constituents in Jitai Tablets by High-Performance Liquid Chromatography/Diode-Array Detection Coupled with Electrospray Ionization Tandem Mass Spectrometry. Molecules. 2012; 17(9):10470-10493. https://doi.org/10.3390/molecules170910470
Chicago/Turabian StyleWang, Shuping, Lei Liu, Lingling Wang, Yaohua Hu, Weidong Zhang, and Runhui Liu. 2012. "Structural Characterization and Identification of Major Constituents in Jitai Tablets by High-Performance Liquid Chromatography/Diode-Array Detection Coupled with Electrospray Ionization Tandem Mass Spectrometry" Molecules 17, no. 9: 10470-10493. https://doi.org/10.3390/molecules170910470




