Sulfation and Enhanced Antioxidant Capacity of an Exopolysaccharide Produced by the Medicinal Fungus Cordyceps sinensis
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Sulfation of EPS-1

| EPS fraction | CSA:Pyr(v/v) | Yield (mg) a | DS | Mwb (kDa) | Mnb (kDa) | P b (Mw/ Mn) |
|---|---|---|---|---|---|---|
| EPS-1 | — | — | — | 38.0 | 25.9 | 1.47 |
| SEPS-1A | 1:8 | 197 | 0.25 | 17.1 | 16.4 | 1.04 |
| SEPS-1B | 1:4 | 216 | 0.46 | 13.2 | 12.1 | 1.09 |
| SEPS-1C | 1:2 | 229 | 0.93 | 9.4 | 7.8 | 1.20 |
| SEPS-1D | 1:1 | 247 | 1.38 | 4.1 | 3.9 | 1.05 |
2.2. IR Spectra of EPS-1 and SEPS-1
2.3. Changes in Molecular Weight and Chain Conformation

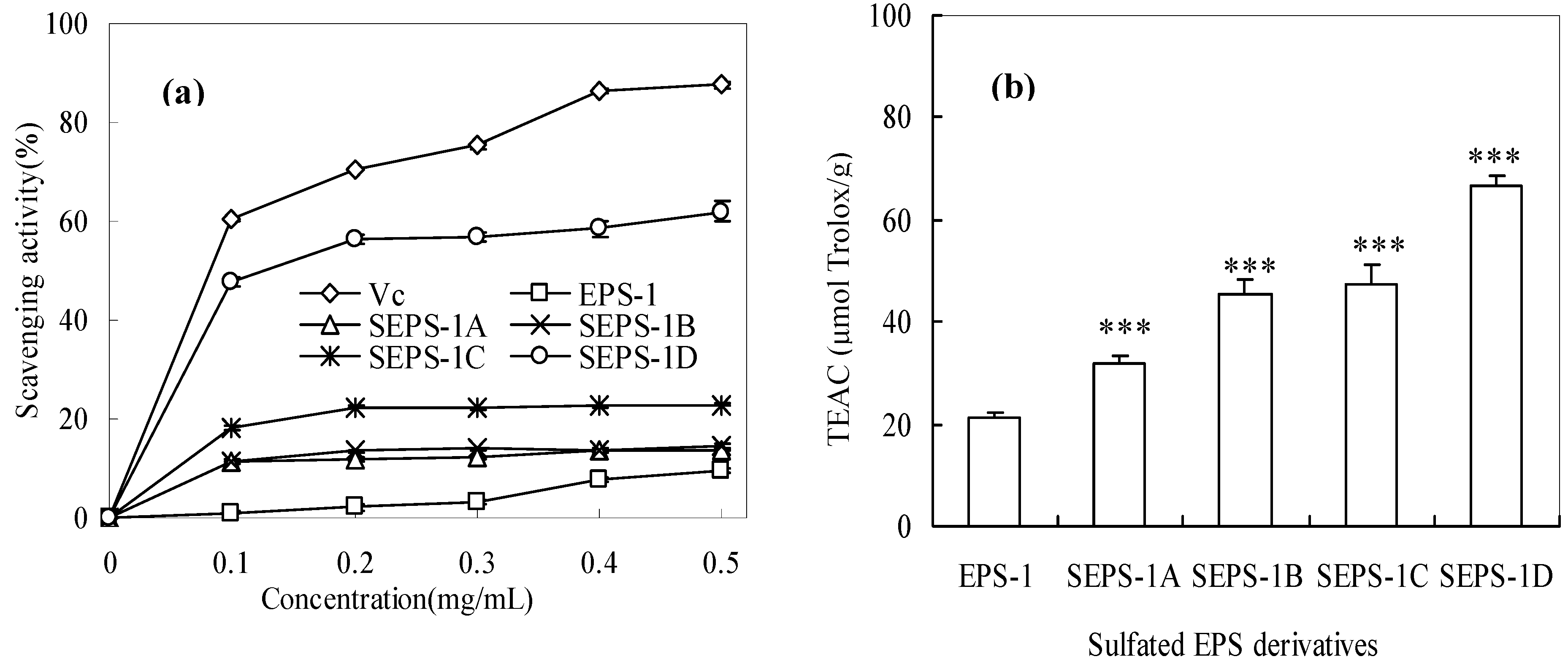
2.4. Antioxidant Activities of Sulfated EPS-1 Derviatives

3. Experimental
3.1. Materials and Chemicals
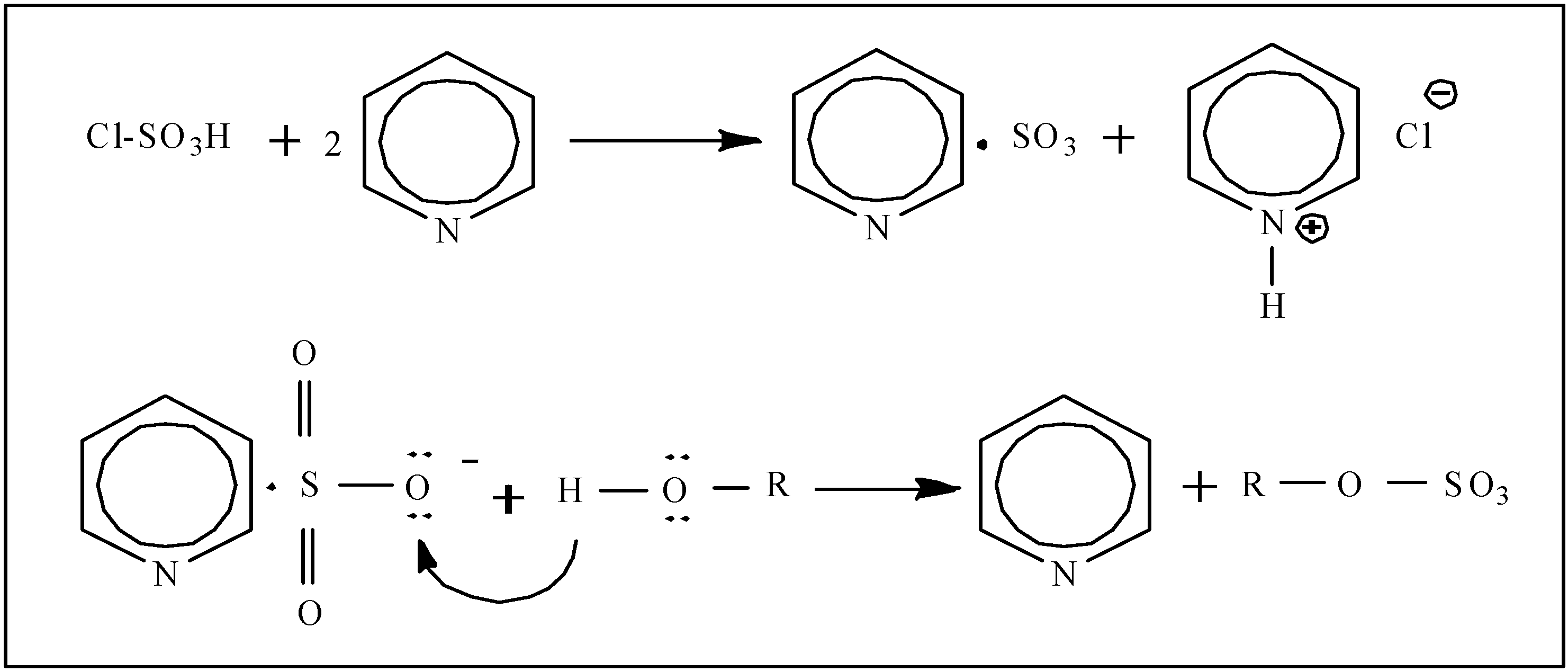
3.2. Sulfation of EPS-1
3.3. Analysis of EPS-1 and SEPS-1 Molecular Properties
3.4. Antioxidant Activity Assays
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Blander, G.; de Oliveira, R.M.; Conboy, C.M.; Haigis, M.; Guarente, L. Superoxide dismutase 1 knock-down induces senescence in human fibro-blasts. J. Biol. Chem. 2003, 278, 38966–38969. [Google Scholar]
- Marx, J.L. Oxygen free radicals linked to many diseases. Science 1987, 235, 529–531. [Google Scholar]
- Willcox, J.K.; Ash, S.L.; Catignani, G.L. Antioxidants and prevention of chronic disease. Crit. Rev. Food Sci. Nutr. 2004, 44, 275–295. [Google Scholar] [CrossRef]
- Kim, K.W.; Thomas, R.L. Antioxidative activity of chitosans with varying molecular weights. Food Chem. 2007, 101, 308–313. [Google Scholar] [CrossRef]
- Carlucci, M.J.; Pujol, C.A.; Ciancia, M.; Noseda, M.D.; Matulewicz, M.C.; Damonte, E.B.; Cerezo, A.S. Antiherpetic and anticoagulant properties of carrageenans from the red seaweed Gigartina skottsbergii and their cyclized derivatives: Correlation between structure and biological activity. Int. J. Biol. Macromol. 1997, 20, 97–105. [Google Scholar] [CrossRef]
- Parish, C.R.; Freeman, C.; Brown, K.J.; Francis, D.J.; Cowden, W.B. Identification of sulfated oligosaccharide-based inhibitors of tumor growth and metastasis using novel in vitro assays for angiogenesis and heparanase activity. Cancer Res. 1999, 59, 3433–3441. [Google Scholar]
- Yamada, T.; Ogamo, A.; Saito, T.; Uchiyama, H.; Nakagawa, Y. Preparation of O-acylated low-molecular-weight carrageenans with potent anti-HIV activity and low anticoagulant effect. Carbohyd. Polym. 2000, 41, 115–120. [Google Scholar] [CrossRef]
- Hu, T.T.; Liu, D.; Chen, Y.; Wu, J.; Wang, S.S. Antioxidant activity of sulfated polysaccharide fractions extracted from Undaria pinnitafida in vitro. Int. J. Biol. Macromol. 2010, 46, 193–198. [Google Scholar] [CrossRef]
- Martinichen-Herrero, J.C.; Carbonero, E.R.; Sassaki, G.L.; Gorin, P.A.J.; Iacomini, M. Anticoagulant and antithrombotic activities of a chemically sulfated galactoglucomannan obtained from the lichen Cladoniaibitipocae. Int. J. Biol. Macromol. 2005, 35, 97–102. [Google Scholar] [CrossRef]
- Liu, Y.H.; Liu, C.H.; Tan, H.N.; Zhao, T.; Cao, J.C.; Wang, F.S. Sulphation of a polysaccharide obtained from Phellinus ribis and potential biological activities of the sulphated derivatives. Carbohyd. Polym. 2009, 77, 370–375. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biot. 2002, 60, 258–274. [Google Scholar] [CrossRef]
- Li, S.P.; Tsim, K.W.K. The Biological and Pharmacological Properties of Cordyceps sinensis, a Traditional Chinese Medicine that has Broad Clinical Applications. In Herbal and Traditional Medicine: Molecular Aspects of Health; Packer, L., Ong, C.N., Halliwell, B., Eds.; Marcel Dekker: New York, NY, USA, 2004; pp. 657–683. [Google Scholar]
- Leung, P.H.; Zhang, Q.X.; Wu, J.Y. Mycelium cultivation, chemical composition and antitumor activity of a Tolypocladium sp. fungus isolated from wild Cordyceps sinensis. J. Appl. Microbiol. 2006, 101, 275–283. [Google Scholar] [CrossRef]
- Leung, P.H.; Zhao, S.N.; Ho, K.P.; Wu, J.Y. Chemical properties and antioxidant activity of exopolysaccharides from mycelial culture of Cordyceps sinensis fungus Cs-HK1. Food Chem. 2009, 114, 1251–1256. [Google Scholar] [CrossRef]
- Mähner, C.; Lechner, M.D.; Nordmeier, E. Synthesis and characterisation of dextran and pullulan sulphate. Carbohyd. Res. 2001, 331, 203–208. [Google Scholar] [CrossRef]
- Mollet, J.C.; Rahaoui, A.; Lemoine, Y. Yield, chemical composition and gel strength of agarocolloids of Gracilaria gracilis, Gracilariopsis longissima and the newly reported Gracilaria cf. vermiculophylla from Roscoff (Brittany, France). J. Appl. Phycol. 1998, 10, 59–66. [Google Scholar]
- Whistler, R.L.; Spencer, W.W. Preparation and properties of several polysaccharide sulfates. Arch. Biochem. Biophys. 1961, 95, 36–41. [Google Scholar] [CrossRef]
- Tao, Y.Z.; Zhang, L.; Peter, C.K. Physicochemical properties and antitumor activities of water-soluble native and sulfated hyperbranched mushroom polysaccharides. Carbohydr. Res. 2006, 341, 2261–2269. [Google Scholar] [CrossRef]
- Wang, Z.M.; Cheung, Y.C.; Leung, P.H.; Wu, J.Y. Ultrasonic treatment for improved solution properties of a high-molecular weight exopolysaccharide produced by a medicinal fungus. Bioresour. Technol. 2010, 101, 5517–5522. [Google Scholar] [CrossRef]
- Wang, J.L.; Guo, H.Y.; Zhang, J.; Wang, X.F.; Zhao, B.T.; Yao, J.; Wang, Y.P. Sulfated modification, characterization and structure–antioxidant relationships of Artemisia sphaerocephala polysaccharides. Carbohyd. Polym. 2010, 81, 897–905. [Google Scholar] [CrossRef]
- Yang, X.B.; Gao, X.D.; Han, F.; Tan, R.X. Sulfation of a polysaccharide produced by a marine filamentous fungus Phoma herbarum YS4108 alters its antioxidant properties in vitro. Biochim. Biophys. Acta Gen. Subjects 2005, 1725, 120–127. [Google Scholar] [CrossRef]
- Yan, J.K.; Li, L.; Wang, Z.M.; Leung, P.H.; Wang, W.Q.; Wu, J.Y. Acidic degradation and enhanced antioxidant activities of exopolysaccharides from Cordyceps sinensis mycelial culture. Food Chem. 2009, 117, 641–646. [Google Scholar] [CrossRef]
- Simic, M.G.; Jovanovic, S.V.; Niki, E. Mechanisms of lipid oxidative processes and their inhibition. In Lipid Oxidation in Food, ACS Symposium Series 500; St. Angelo, A.J., Ed.; American Chemistry Society: Washington, DC, USA, 1992; pp. 14–32. [Google Scholar]
- Jovanovic, S.V.; Jancovic, I.; Josimovic, L. Electron-transfer reactions of alkyperoxy radicals. J. Am. Chem. Soc. 1992, 114, 9018–9021. [Google Scholar] [CrossRef]
- Kishy, Y.F.M.; Al-Sayed, H.M.A. Free-radical scavenging and antioxidative activities of some polysaccharides in emulsions. LWT Food Sci. Technol. 2007, 40, 270–277. [Google Scholar] [CrossRef]
- Tsiapali, E.; Whaley, S.; Kalbfleisch, J.; Ensley, H.E.; Browder, I.W.; Williams, D.L. Glucans exhibit weak antioxidant activity, but stimulate macrophage free radical activity. Free Radic. Biol. Med. 2001, 30, 393–402. [Google Scholar] [CrossRef]
- Chen, S.K.; Tsai, M.L.; Huang, J.R.; Chen, R.H. In vitro antioxidant activities of low-molecular-weight polysaccharides with various functional groups. J. Agric. Food Chem. 2009, 57, 2699–2704. [Google Scholar] [CrossRef]
- Beck, R.H.; Fitton, M.G.; Kricheldorf, H.R. Chemical Modification of Polysaccharides. In Handbook of Polymer Synthesis; Kricheldorf, H.R., Ed.; Marcel Dekker: New York, NY, USA, 1992; p. 1538. [Google Scholar]
- Wang, L.; Li, X.X.; Chen, Z.H. Sulfated modification of the polysaccharides obtained from defatted rice bran and their antitumor activities. Int. J. Biol. Macromol. 2009, 44, 211–214. [Google Scholar] [CrossRef]
- Ogawa, K.; Hatano, M. Circular dichroism of the complex of a (1-3)-β-D-glucan with Congo Red. Carbohyd. Res. 1978, 67, 527–535. [Google Scholar] [CrossRef]
- He, Z.S.; Luo, H.; Cao, C.H.; Cui, Z.W. Photometric determination of hydroxyl free radical in Feonton system by brilliant green. Am. J. Chin. Clin. Med. 2004, 6, 236–243. [Google Scholar]
- Sample Availability: Samples of the compounds EPS and EPS-1 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yan, J.-K.; Wang, W.-Q.; Ma, H.-L.; Wu, J.-Y. Sulfation and Enhanced Antioxidant Capacity of an Exopolysaccharide Produced by the Medicinal Fungus Cordyceps sinensis. Molecules 2013, 18, 167-177. https://doi.org/10.3390/molecules18010167
Yan J-K, Wang W-Q, Ma H-L, Wu J-Y. Sulfation and Enhanced Antioxidant Capacity of an Exopolysaccharide Produced by the Medicinal Fungus Cordyceps sinensis. Molecules. 2013; 18(1):167-177. https://doi.org/10.3390/molecules18010167
Chicago/Turabian StyleYan, Jing-Kun, Wen-Qiang Wang, Hai-Le Ma, and Jian-Yong Wu. 2013. "Sulfation and Enhanced Antioxidant Capacity of an Exopolysaccharide Produced by the Medicinal Fungus Cordyceps sinensis" Molecules 18, no. 1: 167-177. https://doi.org/10.3390/molecules18010167
APA StyleYan, J.-K., Wang, W.-Q., Ma, H.-L., & Wu, J.-Y. (2013). Sulfation and Enhanced Antioxidant Capacity of an Exopolysaccharide Produced by the Medicinal Fungus Cordyceps sinensis. Molecules, 18(1), 167-177. https://doi.org/10.3390/molecules18010167







