Chemical Composition and Biological Activity of Essential Oils of Origanum vulgare L. subsp. vulgare L. under Different Growth Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results of Field Experiment
| Measured parameters | Rows | Biological phases | |||||
|---|---|---|---|---|---|---|---|
| Growth (April 19th) | Bloom beg. (May 24th) | Full bloom (June 18th) | |||||
| Vegetation height (cm) | single | 14.2 | b | 53.8 | b | 84.2 | a |
| binate | 18.3 | a | 62.4 | a | 75.6 | a | |
| Row width | single | 44.8 | b | 46.2 | b | 45.6 | b |
| (cm) | binate | 70.7 | a | 109.5 | a | 106.6 | a |
| PAR absorbance | single | 26 | b | 100 | a | 96 | b |
| (%) | binate | 18 | a | 100 | a | 98 | a |
| Fresh biomass | single | 192 | b | 422 | b | 569 | b |
| (g per plant) | binate | 289 | a | 662 | a | 696 | a |
| Fresh biomass | single | 963 | a | 2111 | a | 2846 | a |
| (g m−2) | binate | 830 | b | 1921 | b | 2020 | b |
| Essential oil | single | 0.007 | a | 0.018 | a | 0.030a | b |
| (% fresh biomass) | binate | 0.009 | a | 0.012 | a | 0.051a | a |
| Essential oil samples | Plant disposal | Single row | Binate row | ||||
|---|---|---|---|---|---|---|---|
| Oregano biomass | Fresh | Dried | Fresh | Dried | |||
| RI a | RI b | Component | Id. c | 1% d | % 2 d | % 3 d | % 4 d |
| Monoterpene hydrocarbons | 18.1 | 14.0 | 19.4 | 16.7 | |||
| 931 | 1023 | α-Thujene | 1, 2 | 0.2 | 0.2 | t | 0.3 |
| 938 | 1032 | α-Pinene | 1, 2, 3 | 0.5 | 0.3 | 0.1 | 0.4 |
| 973 | 1132 | Sabinene | 1, 2 | 9.1 | 4.8 | 2.5 | 3.2 |
| 993 | 1174 | Myrcene | 1, 2, 3 | 0.5 | 0.7 | 1.2 | 0.6 |
| 1001 | 1146 | δ2-Carene | 1, 2 | 0.3 | 0.5 | 0.2 | 1.1 |
| 1005 | 1150 | α-Phellandrene | 1, 2, 3 | 0.5 | |||
| 1012 | 1189 | α-Terpinene | 1, 2, 3 | t | |||
| 1020 | 1187 | o-Cymene | 1, 2 | 0.2 | |||
| 1025 | 1278 | p-Cymene | 1, 2, 3 | 3.9 | 3.8 | 3.3 | 4.1 |
| 1029 | 1218 | β-Phellandrene | 1, 2 | 0.7 | |||
| 1030 | 1203 | Limonene | 1, 2, 3 | 0.8 | |||
| 1038 | 1245 | (Z)-β-Ocimene | 1, 2 | 0.8 | 0.8 | 4.0 | 1.9 |
| 1049 | 1262 | (E)-β-Ocimene | 1, 2 | 0.9 | 0.8 | 3.1 | 1.5 |
| 1057 | 1256 | γ-Terpinene | 1, 2, 3 | 0.3 | 0.6 | 0.9 | 1.5 |
| 1086 | 1265 | Terpinolene | 1, 2, 3 | 0.1 | 0.2 | 0.1 | 0.4 |
| 1129 | 1386 | allo-Ocimene | 1, 2 | 1.1 | 1.1 | 4.0 | 0.6 |
| Oxygenated monoterpenes | 7.2 | 15.5 | 3.9 | 17.6 | |||
| 1034 | 1213 | 1,8-Cineole | 1, 2, 3 | 0.6 | 3.9 | 2.6 | |
| 1059 | 1555 | (Z)-Sabinyl acetate | 1, 2 | 0.4 | 0.2 | 0.1 | 0.6 |
| 1076 | 1450 | (Z)-Linalool oxide (furanoid) | 1, 2 | 0.1 | t | 0.3 | |
| 1093 | 1474 | trans-Sabinene hydrate | 1, 2 | 0.1 | 0.2 | t | 0.7 |
| 1098 | 1553 | Linalool | 1, 2, 3 | 0.7 | 0.9 | 0.8 | 2.0 |
| 1117 | 1571 | trans-p-Menth-2-en-1-ol | 1, 2 | 0.2 | 0.3 | 0.1 | 0.6 |
| 1128 | 1638 | cis-p-Menth-2-en-1-ol | 1, 2 | t | 0.4 | 0.6 | |
| 1141 | 1684 | trans-Verbenol | 1, 2 | 0.1 | |||
| 1145 | 1532 | Camphor | 1, 2, 3 | t | |||
| 1155 | 1652 | Sabina ketone | 1, 2 | 0.3 | 0.5 | ||
| 1167 | 1719 | Borneol | 1, 2, 3 | 0.2 | 0.1 | 0.4 | |
| 1175 | 1583 | cis-Isopulegone | 1, 2 | 0.4 | t | 0.3 | 0.2 |
| 1176 | 1611 | Terpinen-4-ol | 1, 2, 3 | 3.5 | 5.6 | 1.9 | 5.0 |
| 1183 | 1758 | cis-Piperitol | 1, 2 | 0.1 | 0.1 | ||
| 1189 | 1706 | α-Terpineol | 1, 2, 3 | 0.9 | 2.3 | 0.6 | 2.3 |
| 1193 | 1648 | Myrtenal | 1, 2 | 0.1 | |||
| 1196 | 1804 | Myrtenol | 1, 2 | 0.2 | 0.3 | ||
| 1207 | 1689 | trans-Piperitol | 1, 2 | 0.1 | 0.1 | 0.2 | |
| 1218 | 1802 | Cuminaldehyde | 1, 2 | 0.2 | |||
| 1238 | 1662 | Pulegone | 1, 2 | 0.1 | 0.8 | ||
| 1242 | 1752 | Carvone | 1, 2 | 0.2 | t | ||
| 1265 | 1680 | cis-Piperitone oxide | 1, 2 | 0.1 | |||
| 1275 | 1744 | Phellandral | 1, 2 | 0.1 | 0.1 | ||
| 1288 | 2113 | Cumin alcohol | 1, 2 | 0.1 | 0.2 | ||
| 1315 | 2073 | p-Mentha-1,4-dien-7-ol | 1, 2 | 0.1 | |||
| Sesquiterpene hydrocarbons | 34.2 | 22.9 | 43.9 | 16.5 | |||
| 1339 | 1494 | Bicycloelemene | 1, 2 | 0.2 | 0.5 | 1.8 | 0.5 |
| 1348 | 1466 | α-Cubebene | 1, 2 | 0.1 | 0.1 | 0.2 | |
| 1377 | 1497 | α-Copaene | 1, 2 | 0.2 | 0.2 | 0.2 | 0.9 |
| 1382 | 1547 | β-Cubebene | 1, 2 | 0.2 | |||
| 1385 | 1535 | β-Bourbonene | 1, 2 | 0.7 | 1.5 | 1.1 | 1.0 |
| 1387 | 1600 | β-Elemene | 1, 2 | 0.6 | 0.3 | 0,8 | 0.2 |
| 1408 | 1529 | α-Gurjunene | 1, 2 | 0.1 | 0.1 | ||
| 1415 | 1612 | β-Caryophyllene | 1, 2, 3 | 15.6 | 8.8 | 17.2 | 5.3 |
| 1432 | 1612 | β-Gurjunene | 1, 2 | 0.3 | |||
| 1432 | 1650 | γ-Elemene | 1, 2 | 1.3 | |||
| 1398 | 1685 | α-Elemene | 1, 2 | t | |||
| 1437 | 1628 | Aromadendrene | 1, 2 | 0.1 | 0.1 | ||
| 1438 | 1573 | trans-α-Bergamotene | 1, 2 | 1.7 | 0.3 | ||
| 1452 | 1673 | (E)-β-Farnesene | 1, 2 | t | |||
| 1455 | 1689 | α-Humulene | 1, 2 | 2.1 | 1.1 | 2.8 | 0.8 |
| 1463 | 1667 | allo-Aromadendrene | 1, 2 | 0.5 | 0.8 | 0.8 | 0.7 |
| 1477 | 1726 | Germacrene D | 1, 2 | 4.5 | 3.3 | 9.8 | 2.1 |
| 1478 | 1679 | α-Amorphene | 1, 2 | t | |||
| 1478 | 1704 | γ-Muurolene | 1, 2 | 0.4 | 0.4 | 0.8 | |
| 1483 | 1784 | ar-Curcumene | 1, 2 | t | |||
| 1489 | 1734 | epi-Bicyclosesquiphellandrene | 1, 2 | 1.0 | 0.8 | 1.9 | 0.7 |
| 1490 | 1694 | cis β-Guajene | 1, 2 | 0.1 | 0.1 | 0.2 | 0.2 |
| 1492 | 1756 | Bicyclogermacrene | 1, 2 | 0.8 | 2.9 | 0.1 | |
| 1494 | 1740 | Valencene | 1, 2 | t | |||
| 1503 | 1740 | α-Muurolene | 1, 2 | 0.6 | 0.3 | 0.7 | 0.7 |
| 1506 | 1758 | (E,E)-α-Farnesene | 1, 2 | 2.6 | 0.3 | 0.6 | |
| 1510 | 1743 | β-Bisabolene | 1, 2 | 0.7 | 0.2 | ||
| 1515 | 1776 | γ-Cadinene | 1, 2 | 1.4 | 1.3 | 0.5 | |
| 1519 | 1839 | 1-S-cis-Calamenene | 1, 2 | t | |||
| 1526 | 1773 | δ-Cadinene | 1, 2 | 2.4 | 1.7 | 0.2 | 1.0 |
| 1532 | 1745 | α-Cadinene | 1, 2 | 0.2 | 0.2 | 0.3 | 0.1 |
| 1541 | 1918 | α-Calacorene | 1, 2 | 0.1 | 0.1 | ||
| 1544 | 1854 | Germacrene B | 1, 2 | 0.7 | |||
| Oxygenated sesquiterpenes | 16.7 | 24.9 | 20.6 | 20.0 | |||
| 1520 | 2048 | endo-1-Bourbonanol | 1, 2 | 0.2 | 0.3 | ||
| 1553 | 2076 | cis-α-Copaen-8-ol | 1, 2 | 0.4 | |||
| 1565 | 2057 | Ledol | 1, 2 | 0.3 | 0.4 | 0.2 | 0.6 |
| 1575 | 2069 | Germacrene D 4-ol | 1, 2 | t | t | ||
| 1577 | 2150 | Spathulenol | 1, 2, 3 | 6.1 | 18.6 | 5.3 | 9.4 |
| 1579 | 2008 | Caryophyllene oxide | 1, 2, 3 | 0.2 | 1.4 | 1.1 | 2.9 |
| 1587 | 2098 | Globulol | 1, 2 | 0.8 | 1.0 | 1.0 | |
| 1591 | 2104 | Viridiflorol | 1, 2 | 0.8 | 0.7 | 1.0 | 0.6 |
| 1621 | 2324 | Caryophylladienol II | 1, 2 | 0.4 | |||
| 1636 | 2183 | γ-Eudesmol | 1, 2 | 5.0 | 6.7 | 1.0 | |
| 1638 | 2316 | Caryophylladienol I | 1, 2 | 0.6 | |||
| 1640 | 2158 | t-Cadinol | 1, 2 | 1.0 | 0.7 | 2.1 | 0.8 |
| 1642 | 2209 | t-Muurolol | 1, 2 | 1.6 | 3.2 | 0.6 | |
| 1645 | 2208 | Torreyol | 1, 2 | 0.7 | 0.4 | ||
| 1650 | allo-Aromadendrene oxide I | 1, 2 | 0.4 | ||||
| 1652 | 2255 | α-Cadinol | 1, 2 | 1.7 | 2.0 | ||
| Phenols | 1.4 | 12.5 | 0.2 | 16.6 | |||
| 1239 | 1609 | Thymol methyl ether | 1, 2, 3 | t | 0.3 | ||
| 1245 | 1975 | Carvacrol methyl ether | 1, 2, 3 | 0.2 | 0.9 | ||
| 1293 | 2198 | Thymol | 1, 2, 3 | 0.5 | 0.1 | 1.1 | |
| 1299 | 2239 | Carvacrol | 1, 2, 3 | 1.4 | 11.7 | 0.1 | 14.3 |
| 1353 | 2186 | Eugenol | 1, 2, 3 | 0.1 | |||
| Carbonylic compounds | 0.1 | 0.4 | 0.5 | 0.6 | |||
| 975 | 1312 | 1-Octen-3-one | 1, 2 | 0.1 | 0.5 | 0.3 | |
| 1085 | 1690 | Cryptone | 1, 2 | t | 0.1 | ||
| 1395 | 1969 | cis-Jasmone | 1, 2 | 0.1 | |||
| 1482 | 1958 | (E)-β-Ionone | 1, 2, 3 | 0.2 | |||
| 1835 | 2131 | Hexahydrofarnesyl acetone | 1, 2 | 0.1 | t | 0.2 | |
| Hydrocarbons | 14.5 | 1.2 | 0.8 | 3.5 | |||
| 800 | 800 | Octane | 1, 2, 3 | t | |||
| 2100 | 2100 | Heneicosane | 1, 2, 3 | 0.2 | |||
| 2200 | 2200 | Docosane | 1, 2, 3 | 0.1 | t | ||
| 2300 | 2300 | Tricosane | 1, 2, 3 | 0.4 | 0.2 | ||
| 2400 | 2400 | Tetracosane | 1, 2, 3 | 0.4 | 0.1 | t | 0.2 |
| 2500 | 2500 | Pentacosane | 1, 2, 3 | 0.8 | 0.2 | 0.1 | 0.4 |
| 2600 | 2600 | Hexacosane | 1, 2, 3 | 1.1 | 0.1 | t | 0.2 |
| 2700 | 2700 | Heptacosane | 1, 2, 3 | 1.5 | 0.2 | 0.1 | 0.5 |
| 2800 | 2800 | Octacosane | 1, 2, 3 | 1.7 | 0.1 | 0.1 | 0.4 |
| 2900 | 2900 | Nonacosane | 1, 2, 3 | 2.5 | 0.2 | 0.2 | 0.5 |
| 3000 | 3000 | Triacontane | 1, 2, 3 | 2.2 | t | 0.1 | 0.4 |
| 3100 | 3100 | Entriacontane | 1, 2, 3 | 1.7 | 0.2 | 0.2 | 0.4 |
| 3200 | 3200 | Dotriacontane | 1, 2, 3 | 1.1 | t | t | 0.2 |
| 3300 | Tritriacontane | 1, 2 | 0.5 | 0.1 | t | 0.1 | |
| 3400 | Tetratriacontane | 1, 2 | 0.2 | t | |||
| 3500 | Pentatriacontane | 1, 2 | 0.1 | t | |||
| Others | 1.0 | 2.5 | 1.1 | 1.7 | |||
| 980 | 1454 | 1-Octen-3-ol | 1, 2, 3 | 0.4 | 1.3 | 0.4 | 1.2 |
| 992 | 1394 | Octan-3-ol | 1, 2, 3 | 0.1 | 0.3 | ||
| 1286 | 1485 | Dihydroedulan II | 1, 2 | 0.3 | 0.9 | 0.4 | 0.5 |
| 1950 | 2622 | (Z)-Phytol | 1, 2 | 0.2 | 0.3 | T | |
| 1957 | 2931 | Hexadecanoic acid | 1, 2, 3 | t | |||
| TOTAL | 93.2 | 93.9 | 90.4 | 93.2 | |||
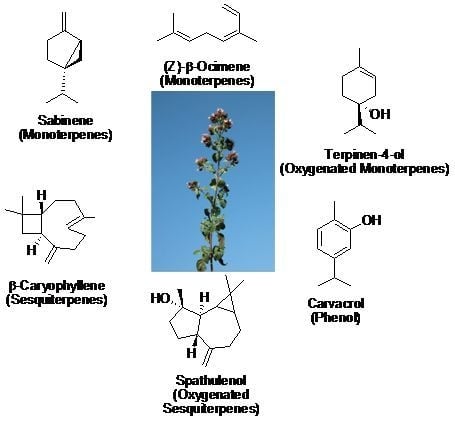
2.2. Chemical Composition of the Essential Oil

2.3. Antimicrobial Activity
| Bacterial strain | Oregano essential oil samples | G | |||
|---|---|---|---|---|---|
| Single row | Binate row | ||||
| Fresh (1) | Dried (2) | Fresh (3) | Dried (4) | ||
| Bacillus cereus ATCC 11778 | 50 (100) | 50 | 50 (100) | 50 | 1.56 |
| Bacillus subtilis ATCC 6633 | 50 (100) | 50 | 50 (100) | 50 | 1.56 |
| Staphylococcus aureus ATCC 2592 | 50 (100) | 50 (100) | 100 | 50 (100) | 3.12 |
| Staphylococcus epidermidis ATCC 12228 | 50 (100) | 50 (100) | 100 | 50 (100) | 6.25 |
| Streptococcus faecalis ATTC 29212 | 100 | 50 (100) | 100 | 50 (100) | >100 |
| Escherichia coli ATCC 25922 | 100 | 50 (100) | 100 | 50 (100) | 3.12 |
| Proteus mirabilis ATCC 25933 | 100 | 100 | >100 | 100 | 100 |
| Proteus vulgaris ATCC 13315 | >100 | 100 | >100 | 100 | 100 |
| Pseudomonas aeruginosa ATCC 27853 | >100 | >100 | >100 | >100 | 12.5 |
| Salmonella typhi Ty2 ATCC 19430 | >100 | >100 | >100 | >100 | >100 |
3. Experimental
3.1. Field Trial
3.2. Essential Oil Extraction and Analysis
3.3. Antimicrobial Assay
4. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- De Feo, V.; de Falco, E.; Nicolella, E.; Roscigno, G. First observation on a collection of aromatic plants in a plain of the Campania region (Southern Italy). Acta Hort. 2005, 723, 441–445. [Google Scholar]
- Marzi, V.; Tedone, L. Fattori climatici e socio economici nell’evoluzione del paesaggio agrario e forestale in ambiente mediterraneo. (in Italian). Ital. J. Agron. 2006, 4 (Suppl.), 23–30. [Google Scholar]
- Gonzáles-Tejero, M.R.; Casares-Porcel, M.; Sánchez-Rojas, C.P.; Ramiro-Gutiérrez, J.M.; Molero-Mesa, J.; Pieroni, A.; Giusti, M.E.; Censorii, E.; de Pasquale, C.; Della, A.; et al. Medicinal plants in the Mediterranean area: Synthesis of the results of the project RUBIA. J. Ethnopharmacol. 2008, 116, 341–357. [Google Scholar] [CrossRef]
- Hadjichambis, A.C.; Paraskeva-Hadjichambi, D.; Della, A.; Giusti, M.E.; de Pasquale, C.; Lenzarini, C.; Censorii, E.; Gonzáles-Tejero, M.E.; Sánchez-Rojas, C.P.; Ramiro-Gutierrez, J.M.; et al. Wild and semi-domesticated food plant consumption in seven circum-Mediterranean areas. Int. J. Food Sci. Nutr. 2008, 5, 383–414. [Google Scholar]
- Senatore, F. (Ed.) Oli Essenziali: Provenienza, Estrazione ed Analisi Chimica; Mediche Scientifiche Internazionali: Roma, Italy, 2000; pp. 115–125.
- Sahin, F.; Gulluce, M.; Daferera, D.; Sokmen, A.; Polissiou, M.; Agar, G. Biological activities of the essential oils and methanol extract of Origanum vulgare subsp. vulgare in the Eastern Anatolia Region of Turkey. Food Control 2004, 15, 549–557. [Google Scholar] [CrossRef]
- Faleiro, L.; Miguel, G.; Gomes, S.; Costa, L.; Venâncio, F.; Teixeira, A.; Figueiredo, C.; Barroso, J.G.; Pedro, L.G. Antibacterial and antioxidant activities of essential oils isolated from Thymbra capitata L. (Cav.) and Origanum vulgareL. J. Agric. Food Chem. 2005, 53, 8162–8168. [Google Scholar] [CrossRef]
- Souza, E.L.; Stamford, T.L.M.; Lima, E.O.; Trajano, V.N. Effectiveness of Origanum vulgare L. essential oil to inhibit the growth of food spoiling yeasts. Food Control 2007, 18, 409–413. [Google Scholar] [CrossRef]
- Saraç, N.; Uğur, A.; Duru, M.E.; Varol, Ö. Antimicrobial activity, antioxidant activity and chemical composition of Origanum onites L. and Origanum vulgare L. subsp. hirtum (Link) Ietswaart from Mugla (Turkey). Acta Hortic. 2009, 826, 397–403. [Google Scholar]
- Coelho da Costa, A.; Cavalcanti dos Santos, B.E.; Santos, F.L.; Lima, E.O. Antibacterial activity of the essential oil of Origanum vulgare L. (Lamiaceae) against bacterial multiresistant strains isolated from nosocomial patients. Rev. Bras. Farmacogn. 2009, 19, 236–241. [Google Scholar] [CrossRef]
- Tommasi, L.; Negro, C.; Miceli, A.; Mazzotta, F. Antimicrobial activity of essential oils from aromatic plants grown in the Mediterranean area. J. Essent. Oil Res. 2009, 21, 185–189. [Google Scholar] [CrossRef]
- Dadalioglu, I.; Evrendilek, G.A. Chemical composition and antibacterial effects of essential oils of Turkish oregano (Origanum minutiflorum), bay laurel (Laurus nobilis), Spanish lavender (Lavandula stoechas L.), and fennel (Foeniculum vulgare) on common foodborne pathogens. J. Agric. Food Chem. 2004, 52, 8255–8260. [Google Scholar] [CrossRef]
- Baydar, H.; Sagdic, O.; Özkan, G.; Karadogan, T. Antibacterial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Control 2004, 15, 169–172. [Google Scholar] [CrossRef]
- Vardar-Ünlü, G.; Ünlü, M.; Dönmez, E.; Vural, N. Chemical composition and in vitro antimicrobial activity of the essential oil of Origanum minutiflorum O Schwarz & PH Davis. J. Sci. Food Agric. 2007, 87, 255–259. [Google Scholar] [CrossRef]
- Bouhdid, S.; Skali, S.N.; Idaomar, M.; Zhiri, A.; Baudoux, D.; Amensour, M.; Abrini, J. Antibacterial and antioxidant activities of Origanum compactum essential oil. Afr. J. Biotechnol. 2008, 7, 1563–1570. [Google Scholar]
- Başer, K. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Design 2008, 14, 3106–3119. [Google Scholar] [CrossRef]
- Kokkini, S. Taxonomy, diversity and distribution of Origanum species. In Proceedings of IPGRI International Workshop on Oregano, Bari, Italy, 8–12 May 1996; Padulosi, S., Ed.; IPGRI: Rome, Italy, 1997; pp. 2–12. [Google Scholar]
- Giuliani, C.; Maggi, F.; Papa, F.; Maleci Bini, L. Congruence of phytochemical and morphological profiles along an altitudinal gradient in Origanum vulgare subsp. vulgare from Venetian Region (NE Italy). Chem. Biodivers. 2013, 10, 569–583. [Google Scholar] [CrossRef]
- Berghold, H.; Wagner, S.; Mandi, M.; Thaller, A.; Muller, M.; Rakowitz, M.; Pasteiner, S.; Boechzelt, H. Yield, content and composition of the essential oil of 5 oregano strains (Origanum vulgare L.) depending on the developmental stage. Z. Arznei Gewuerzpfla 2008, 13(1), 36–43. [Google Scholar]
- Azizi, A.; Yan, F.; Honermeier, B. Herbage yield, essential oil content and composition of three oregano (Origanum vulgare L.) populations as affected by soil moisture regimes and nitrogen supply. Ind. Crop. Prod. 2009, 29, 554–561. [Google Scholar] [CrossRef]
- Bernestein, N.; Chaimovitch, D.; Dudai, N. Effect of irrigation with secondary treated effluent on essential oil, antioxidant activity, and phenolic compounds in oregano and rosemary. Agronomy J. 2009, 101, 1–10. [Google Scholar] [CrossRef]
- Dordas, C. Foliar application of calcium and magnesium improves growth, yield, and essential oil yield of oregano (Origanum vulgare subsp. hirtum). Ind. Crop. Prod. 2009, 29, 599–608. [Google Scholar] [CrossRef]
- Halva, S.; Craker, L.E.; Simon, J.E.; Charles, D. Light quality, growth and essential oil in dill (Anethum graveolens L.). J. Herbs Spices Med. Plants 1992, 1, 59–69. [Google Scholar] [CrossRef]
- Piccaglia, R. Aromatic plants: A world of flavouring compost. Agro-Food Ind. Hi-Tech 1998, 9, 12–15. [Google Scholar]
- Leto, C.; Carrubba, A.; Trapani, P. Effetti della densità d’impianto sulla coltivazione dell’origano (Origanum heracleoticum L.) in due ambienti siciliani. In Proceedings of Coltivazione e miglioramento di piante officinali, Trento, Italy, 2–3 June, 1994; pp. 569–576.
- Ietswaart, J.H. A Taxonomic Revision of the Genus Origanum (Labiatae); Leiden Botanical Series 4; Leiden University Press: The Hague, The Netherlands, 1980; Volume 4, pp. 106–110. [Google Scholar]
- Marzi, V. Agricultural practices or oregano. In Proceedings of IPGRI International Workshop on Oregano, Bari, Italy, 8–12 May 1996; Padulosi, S., Ed.; IPGRI: Rome, Italy, 1997; pp. 61–67. [Google Scholar]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.; Nychas, G.J.E. Study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol, and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef]
- Rhayour, K.; Bouchikhi, T.; Tantaoui-Elaraki, A.; Sendide, K.; Remmal, A. The mechanism of bactericidal action of oregano and clove essential oils and of their phenolic major components. J. Essent. Oil Res. 2003, 15, 286–292. [Google Scholar] [CrossRef]
- Guiducci, M.; Antognoni, A.; Benincasa, P. Movimento del fogliame, intercettazione ed utilizzazione della luce in colture diverse in funzione della disponibilità idrica. (in Italian). Riv. Agron. 1993, 27, 392–397. [Google Scholar]
- Ercoli, E.; Mensuali, A.; Malorgio, F.; Serra, G. Interception of photosynthetically active radiation, growth and production of bush bean (Phaseolus vulgaris L.). Agric. Mediterr. 1992, 122, 215–224. [Google Scholar]
- EDQM. European Pharmacopoeia, 5th ed.; Council of Europe: Strasbourg, France, 2005. [Google Scholar]
- Jennings, W.; Shibamoto, T. Qualitative Analysis of Flavour and Fragrance Volatiles by Glass Capillary Gas Chromatography; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicone and Carbowax 20M phases. J. Chromatogr. 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy, 4th ed.; Allured Publishing Co.: Carol Stream, IL, USA, 2007. [Google Scholar]
- The Mass Spectrometry Data Centre. Eight Peak Index of Mass Spectra; The Royal Society of Chemistry, Nottingham University: Nottingham, UK, 1983. [Google Scholar]
- Barry, A. The Antimicrobial Susceptibility Test: Principles and Practices; Lea and Febiger: Philadelphia, PA, USA, 1976; p. 180. [Google Scholar]
- Sample Availability: Samples of the compounds identified with number 3 in the Identification column, Table 2, are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Falco, E.; Mancini, E.; Roscigno, G.; Mignola, E.; Taglialatela-Scafati, O.; Senatore, F. Chemical Composition and Biological Activity of Essential Oils of Origanum vulgare L. subsp. vulgare L. under Different Growth Conditions. Molecules 2013, 18, 14948-14960. https://doi.org/10.3390/molecules181214948
De Falco E, Mancini E, Roscigno G, Mignola E, Taglialatela-Scafati O, Senatore F. Chemical Composition and Biological Activity of Essential Oils of Origanum vulgare L. subsp. vulgare L. under Different Growth Conditions. Molecules. 2013; 18(12):14948-14960. https://doi.org/10.3390/molecules181214948
Chicago/Turabian StyleDe Falco, Enrica, Emilia Mancini, Graziana Roscigno, Enrico Mignola, Orazio Taglialatela-Scafati, and Felice Senatore. 2013. "Chemical Composition and Biological Activity of Essential Oils of Origanum vulgare L. subsp. vulgare L. under Different Growth Conditions" Molecules 18, no. 12: 14948-14960. https://doi.org/10.3390/molecules181214948
APA StyleDe Falco, E., Mancini, E., Roscigno, G., Mignola, E., Taglialatela-Scafati, O., & Senatore, F. (2013). Chemical Composition and Biological Activity of Essential Oils of Origanum vulgare L. subsp. vulgare L. under Different Growth Conditions. Molecules, 18(12), 14948-14960. https://doi.org/10.3390/molecules181214948