Synthesis and Characterization of Novel Dendrons Bearing Amino-Nitro-Substituted Azobenzene Units and Oligo(ethylene glycol) Spacers: Thermal, Optical Properties, Langmuir Blodgett Films and Liquid-Crystalline Behaviour
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of the Dendrons
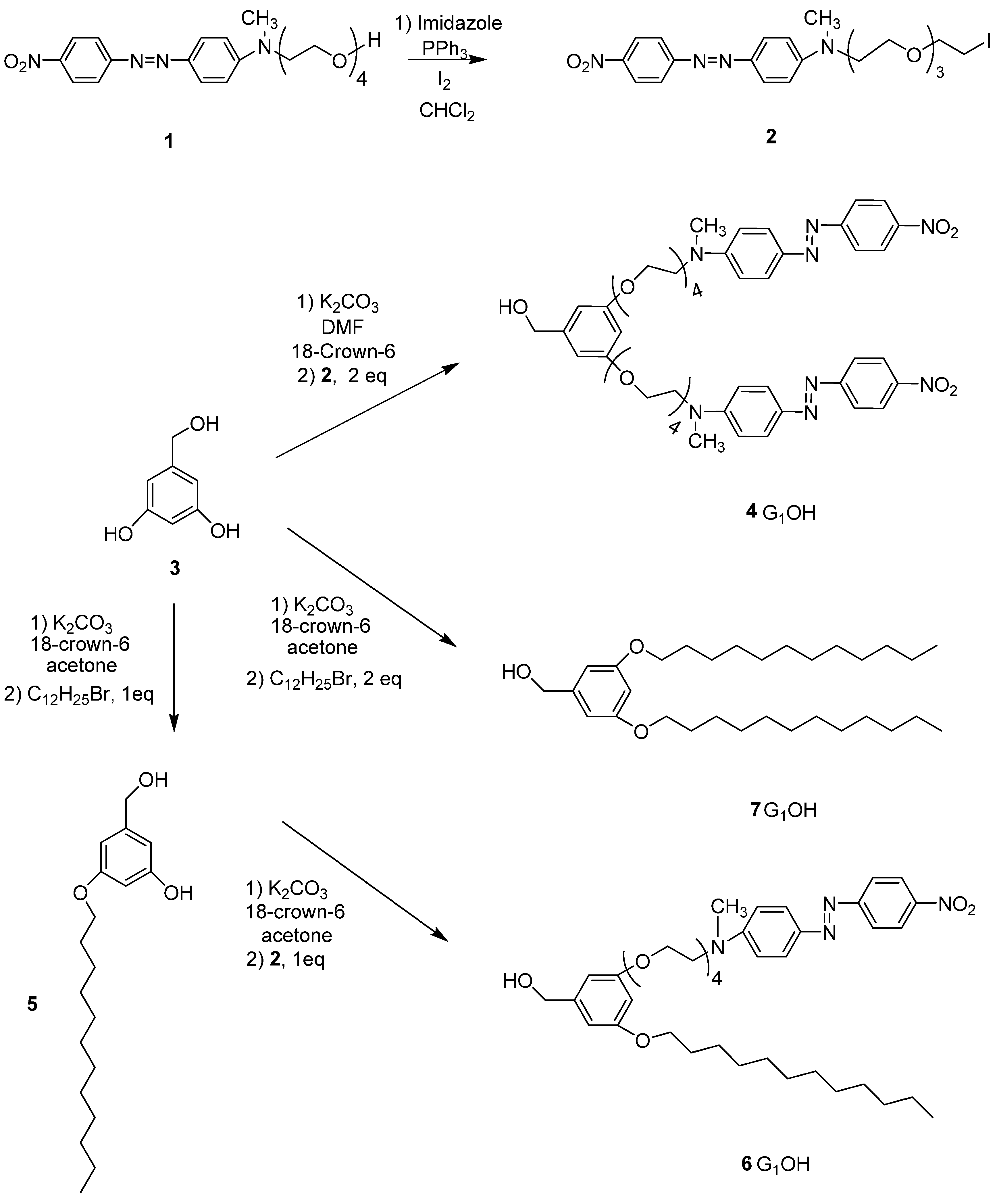

2.2. Characterization of the Dendrons

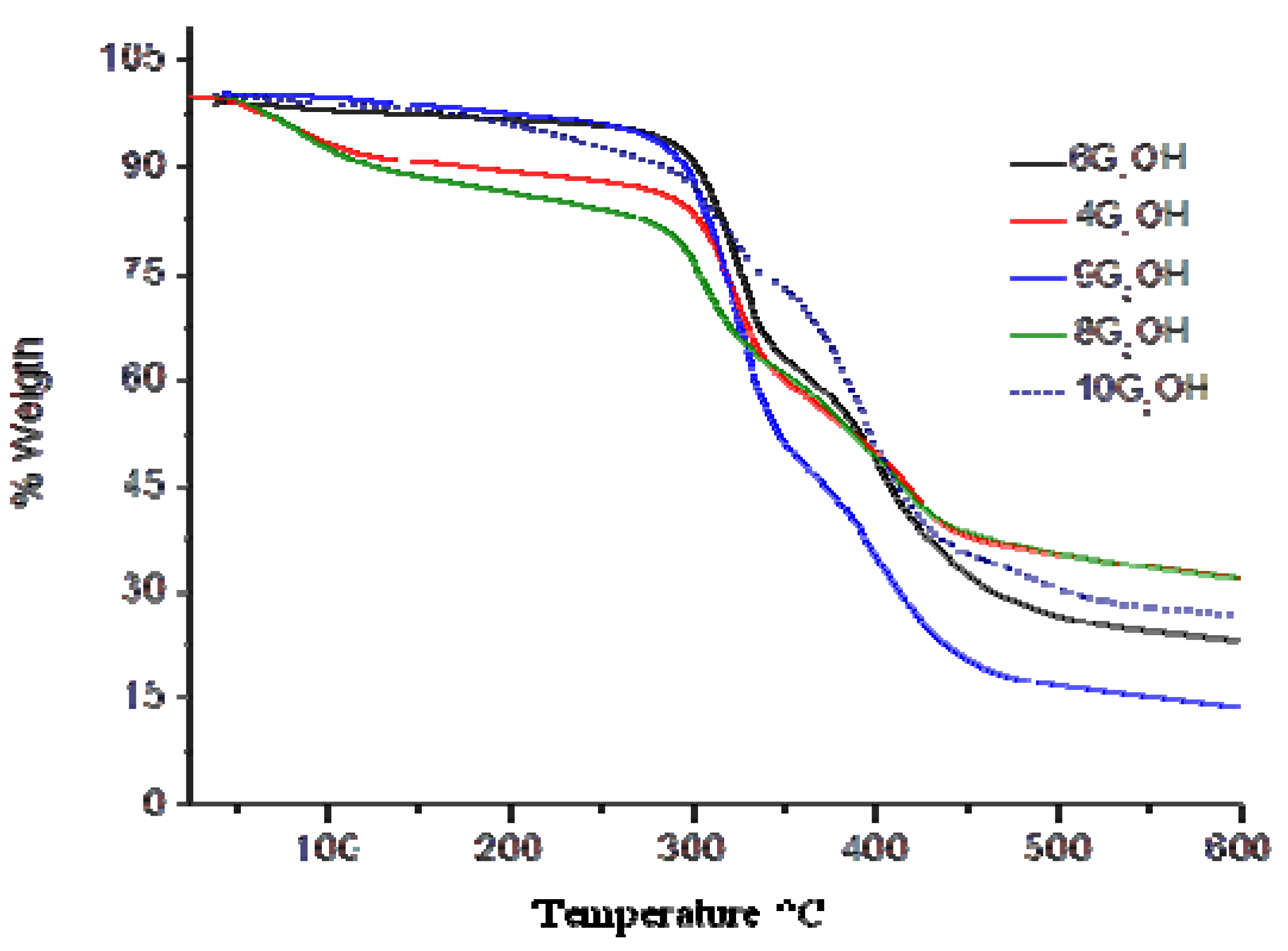
2.3. Thermal Properties of the Dendritic Molecules
| Dendrons | T10 (°C) a | Tm (°C) a | λmax (nm) b | Cut off (nm) b | Dipole Moment μ (D) [M062x] | Dipole Moment μ (D) [BPW91] |
|---|---|---|---|---|---|---|
| 6G1OH | 302 | 36 | 478 | 628 | 14.5253 | 13.9576 |
| 4G1OH | 172 | 71 | 482 | 610 | 9.4828 | 9.1611 |
| 9G2OH | 295 | 29 | 480 | 615 | 29.9523 | 29.4126 |
| 8G2OH | 124 | 68 | 480 | 602 | 17.2420 | 15.5955 |
| 10G2OH | 283 | 51 | 476 | 624 | 17.0627 | 15.7889 |

2.4. Liquid Crystalline Behaviour of the Dendrons

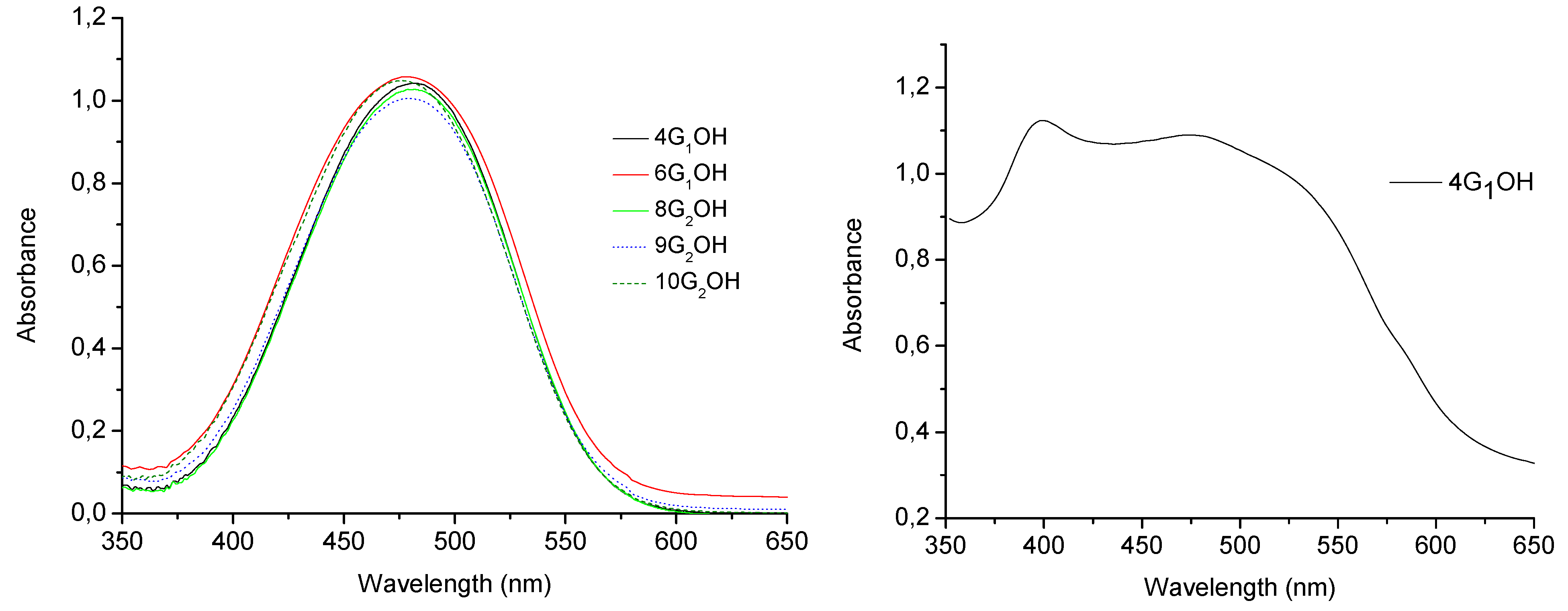
2.5. Optical Properties of the Dendrons
2.6. Theoretical Results

| Dendrons | λ (f) | λ (exp) | Error (%) |
|---|---|---|---|
| 7G1OH | 189 (0.83) | - | - |
| 4G1OH | 469 (1.86) | 482 | 3 |
| 6G1OH | 470 (1.40) | 478 | 3 |
| 8G2OH | 469 (3.07) | 482 | 3 |
| 9G2OH | 469 (2.14) | 480 | 2 |
| 10G2OH | 471 (1.48) | 476 | 1 |
| Dendrons | 7G1OH | 4G1OH | 6G1OH | 8G2OH | 9G2OH | 10G2OH |
|---|---|---|---|---|---|---|
| I | 6.8 | 6.2 | 6.1 | 6.1 | 6.1 | 6.1 |
| A | 0.2 | 3.4 | 3.4 | 3.4 | 3.4 | 3.4 |
| HOMO-LUMO | 7.9 | 4.1 | 4.1 | 4.1 | 4.1 | 4.1 |
| Atom | Atomic Charge | |
|---|---|---|
 | N1 | −1.2 |
| N2 | 0.7 | |
| N3 | −0.7 | |
| N4 | 1.6 |
2.7. Preparation of Langmuir and Langmuir-Blodgett Films


3. Experimental
3.1. General Conditions
3.2. Synthesis of the Dendrons








3.3. Computational Details
3.4. Preparation of Langmuir and Langmuir-Blodgett Films
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the compounds 4G1OH and 6G1OH are available from the authors.
References
- Fréchet, J.M.J.; Tomalia, D. Dendrimers and Other Dendritric Polymers; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Newkome, G.R.; Vögtle, F.; Moorefield, C.N. Dendrimers and Dendrons; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Newkome, G.R.; Shreiner, C.D. Poly(amidoamine), polypropylenimine, and related dendrimers and dendrons possessing different 1/2 branching motifs: An overview of the divergent procedures. Polymer 2008, 49, 1–173. [Google Scholar] [CrossRef]
- Majoral, J.P.; Caminade, A.M. Dendrimers Containing Heteroatoms (Si, P, B, Ge, or Bi). Chem. Rev. 1999, 99, 845–880. [Google Scholar] [CrossRef]
- Vögtle, F.; Richardt, G.; Werner, N. Dendrimer Chemistry; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Momotake, A.; Arai, T. Synthesis, excited state properties, and dynamic structural change of photoresponsive dendrimers. Polymer 2004, 45, 5369–5390. [Google Scholar] [CrossRef]
- Momotake, A.; Arai, T. Photochemistry and photophysics of stilbene dendrimers and related compounds. J. Photochem. Photobiol. C. Photochem. Rev. 2004, 5, 1–25. [Google Scholar] [CrossRef]
- Shibaev, V.; Bobrovsky, A.; Boiko, N. Photoactive liquid crystalline polymer systems with light-controllable structure and optical properties. Prog. Polym. Sci. 2003, 28, 729–836. [Google Scholar] [CrossRef]
- Villavicencio, O.; McGrath, D.V. Advances in dendritic. Macromolecules 2002, 5, 1–44. [Google Scholar]
- Deloncle, R.; Caminade, A.M. Stimuli-responsive dendritic structures: The case of light-driven azobenzene-containing dendrimers and dendrons. J. Photochem. Photobiol. C. Photochem. Rev. 2010, 11, 25–45. [Google Scholar] [CrossRef]
- Mekelburger, H.B.; Rissanen, K.; Vögtle, F. Repetitive-Synthesis of Bulky Dendrimers A reversibly Photoactive Dendrimers with six Azobenzene Side Chains. Chem. Ber. 1993, 126, 1161–1169. [Google Scholar] [CrossRef]
- Buhleier, E.W.; Wehner, W.; Vögtle, F. Cascade and Nonskid-Chain-like Syntheses of molecular Cavity Topologies. Synthesis 1978, 2, 155–158. [Google Scholar]
- Wörner, C.; Mülhaupt, R. Polynitrile and polyamine funtional poly(trimethylene imine) dendrimers. Angew. Chem. Int. Ed. Engl. 1993, 32, 1306–1308. [Google Scholar] [CrossRef]
- De Brabander-van den Berg, E.M.M.; Meijer, E.W. Poly(propylene imine) dendrimers: Large-Scale synthesis by Hetereogeneously catalyzed Hydrogenations. Angew. Chem. Int. Ed. Engl. 1993, 32, 1308–1311. [Google Scholar] [CrossRef]
- Archut, A.; Vögtle, F.; De Cola, L.; Azzellini, G.C.; Balzani, V.; Ramanujam, P.S.; Berg, R.H. Azobenzene-Functionalized Cascade Molecules: Photoswitchable Supramolecular Systems. Chem. Eur. J. 1998, 4, 699–706. [Google Scholar] [CrossRef]
- Dirksen, A.; Zuidema, E.; Williams, R.M.; De Cola, L.; Kauffmann, C.; Vögtle, F.; Roque, A.; Pina, F. Photoactivity and pH Sensitivity of Methyl Orange Functionalized Poly(Propyleneamine) Dendrimers. Macromolecules 2002, 35, 2743–2747. [Google Scholar] [CrossRef]
- Schenning, A.P.; Elissen-Roman, C.; Weener, J.W.; Baars, M.W.; Van der Gaast, S.J.; Meijer, E.W. Amphiphilic Dendrimers as Building Blocks in Supramolecular Assemblies. J. Am. Chem. Soc. 1998, 120, 8199–8208. [Google Scholar] [CrossRef]
- Alcala, R.; Gimenez, R.; Oriol, L.; Pinol, M.; Serrano, J.L.; Villacampa, B.; Vinuales, A.I. Synthesis, Characterization, and Induction of Stable Anisotropy inLiquid Crystalline Photo-addressable PPI Dendrimers. Chem. Mater. 2007, 19, 235–246. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburts-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Hawker, C.J.; Fréchet, J.M.J. Preparation of Polymers with Controlled Molecular Architecture. A New Convergent Approach to Dendritic Macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar] [CrossRef]
- Li, S.; McGrath, D.V. Macromolecular isomers of azobenzene-containing photochromic dendrimers. Polym. Prep. 2000, 41, 861–864. [Google Scholar]
- Li, S.; McGrath, D.V. Effect of Macromolecular Isomerism on the Photomodulation of Dendrimer Properties. J. Am. Chem. Soc. 2000, 122, 6795–6796. [Google Scholar] [CrossRef]
- Nithyanandhan, J.; Jayaraman, N.; Davis, R.; Das, S. Synthesis, Fluorescence and Photoisomerization Studies of Azobenzene-Functionalized Poly(alkyl aryl ether) Dendrimers. Chem. Eur. J. 2004, 10, 689–698. [Google Scholar] [CrossRef]
- Kay, K.Y.; Han, K.J.; Yu, Y.J.; Park, Y.D. Dendritic fullerenes (C60) with photoresponsive azobenzene groups. Tetrahedron Lett. 2002, 43, 5053–5056. [Google Scholar] [CrossRef]
- Rau, H. Photochemistry and Photophysics; Rabek, J.K., Ed.; CRC Press: Boca Raton, FL, USA, 1990; Volume 2, p. 119. [Google Scholar]
- Natansohn, A.; Rochon, P. Macromolecular Science and Engineering Award lecture The Versatility of azobenzene Polymers. Can. J. Chem. 2001, 79, 1093–1100. [Google Scholar]
- Todorov, T.; Nikalova, L.; Tomova, N. Polarization Holography. 1: A new high-efficiency organic material with reversible photoinduced birefringence. Appl. Opt. 1984, 23, 4309–4312. [Google Scholar] [CrossRef]
- Xie, S.; Natansohn, A.; Rochon, P. Recent Developments in Aromatic Azo Polymers Research. Chem. Mater. 1993, 5, 403–411. [Google Scholar] [CrossRef]
- Viswanathan, N.K.; Kim, D.Y.; Bian, S.; Williams, J.; Liu, W.; Li, L.; Samuelson, L.; Kumar, J.; Tripathy, S.K. Surface relief structures on azo polymer films. J. Mater. Chem. 1999, 9, 1941–1955. [Google Scholar] [CrossRef]
- Ichimura, K. Photoalignment of Liquid-Crystal Systems. Chem. Rev. 2000, 100, 1847–1874. [Google Scholar]
- Delaire, J.A.; Nakatani, K. Linear and Nonlinear Optical Properties of Photochromic Molecules and Materials. Chem. Rev. 2000, 100, 1817–1846. [Google Scholar] [CrossRef]
- Natansohn, A.; Rochon, P. Photoinduced Motions in Azo Containing Polymers. Chem. Rev. 2002, 102, 4139–4175. [Google Scholar] [CrossRef]
- Kasha, M. Energy Transfer Mechanisms and the Molecular Exciton Model for Molecular Aggregates. Radiat. Res. 1963, 20, 55–71. [Google Scholar] [CrossRef]
- He, X.; Zhang, H.L.; Yan, D.L.; Wang, X. Synthesis of Side-Chain Liquid-Crystalline Homopolymers and Triblock Copolymers with p-Methoxyazobenzene Moieties and Poly(ethylene glycol) as Coil Segments by Atom Transfer Radical Polymerization and Their Thermotropic Phase Behavior. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2854–2864. [Google Scholar] [CrossRef]
- Tian, Y.Q.; Watanabe, K.; Kong, X.X.; Abe, J.; Iyoda, T. Synthesis, Nanostructures, and Functionality of Amphiphilic Liquid Crystalline Block Copolymers with Azobenzene Moieties. Macromolecules 2002, 35, 3739–3747. [Google Scholar] [CrossRef]
- Saito, M.; Shimomura, T.; Okumura, Y.; Ito, K.; Hayakawa, R. Temperature dependence of inclusion-dissociation behavior between molecular nanotubes and linear polymers. J. Chem. Phys. 2001, 114, 1–3. [Google Scholar] [CrossRef]
- Shimomura, T.; Funaki, T.; Ito, K. Circular Dichroism Study of the Inclusion-Dissociation Behavior of Complexes between a Molecular Nanotube and Azobenzene Substituted Linear Polymers. J. Incl. Phenom. Macroc. Chem. 2002, 44, 275–278. [Google Scholar] [CrossRef]
- Zheng, P.J.; Wang, C.; Hu, X.; Tam, K.C.; Li, L. Supramolecular Complexes of Azocellulose and R-Cyclodextrin: Isothermal Titration Calorimetric and Spectroscopic Studies. Macromolecules 2005, 38, 2859–2864. [Google Scholar] [CrossRef]
- Hu, X.; Zheng, P.J.; Zhao, X.Y.; Li, L.; Tam, K.C.; Gan, L.H. Preparation, characterization and novel photoregulated rheological properties of azobenzene functionalized cellulose derivatives and their α-CD complexes. Polymer 2004, 45, 6219–6225. [Google Scholar] [CrossRef]
- Takashima, Y.; Nakayama, T.; Miyauchi, M.; Kawaguchi, Y. Complex Formation and Gelation between Copolymers Containing Pendant Azobenzene Groups and Cyclodextrin Polymers. Chem. Lett. 2004, 33, 890–891. [Google Scholar] [CrossRef]
- Ikeda, T.; Ooya, T.; Yui, N. Regulation of Pseudo-Polyrotaxane Formation between α-Cyclodetrins and Azobenzene-terminated poly(ethylene glycol). Polym. J. 1999, 31, 658–663. [Google Scholar] [CrossRef]
- Tung, C.H.; Wu, L.Z.; Zhang, L.P.; Chen, B. Supramolecular Systems as Microreactors: Control of Product Selectivity in Organic Phototransformation. Acc. Chem. Res. 2003, 36, 39–47. [Google Scholar] [CrossRef]
- Rivera, E.; Belletête, M.; Natansohn, A.; Durocher, G. Synthesis characterization and optical properties of a novel azo-dye bearing an oligo(ethylene glycol) methyl ether side chain in solution and in the solid state. Can. J. Chem. 2003, 81, 1076–1082. [Google Scholar] [CrossRef]
- Rivera, E.; Carreón-Castro, M.P.; Buendía, I.; Cedillo, G. Optical properties and aggregation of novel azo-dyes bearing an end-capped oligo(ethylene glycol) side chain in solution, solid state and LangmuireBlodgett films. Dyes Pigm. 2006, 68, 217–226. [Google Scholar] [CrossRef]
- Rivera, E.; Carreón-Castro, M.P.; Salazar, R.; Huerta, G.; Becerril, C.; Rivera, L. Preparation and characterization of novel grafted polyethylene based azo-polymers bearing oligo(ethylene glycol) spacers. Polymer 2007, 48, 3420–3428. [Google Scholar] [CrossRef]
- García, T.; Carreón-Castro, M.P.; Gelover-Santiago, A.; Ponce, P.; Romero, M.; Rivera, E. Synthesis and Characterization of Novel Amphiphilic Azo-Polymers Bearing Well-Defined Oligo(Ethylene Glycol) Spacers. Des. Monomers Polym. 2012, 15, 159–174. [Google Scholar] [CrossRef]
- Caicedo, C.; Rivera, E.; Valdez-Hernández, Y.; Carreón-Castro, M.P. Synthesis and characterization of novel liquid-crystalline azo-dyes bearing two amino-nitro substituted azobenzene units and a well-defined, oligo(ethylene glycol) spacer. Mater. Chem. Phys. 2011, 130, 471–480. [Google Scholar] [CrossRef]
- Alvarez-Venicio, V.; Jiménez-Nava, B.; Carreón-Castro, M.P.; Rivera, E.; Méndez, I.A.; Huerta, A.A.; Gutiérrez-Nava, M. Synthesis and incorporation in Langmuir films of oligophenylenevinylene dendrimers bearing a polar head group and different dendritic poly(benzyl ether) branches. Polymer 2008, 49, 3911–3922. [Google Scholar] [CrossRef]
- Kohn, W.; Becke, A.D.; Parr, R.G. Density Functional Theory of Electronic Structure. J. Phys. Chem. 1996, 100, 12974–12980. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. homogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L. self-consistent equations including exchange and correlations effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02. Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P. Electronic Structure of Solids; Ziesche, P., Eschrig, H., Eds.; Akademie Verlag: Berlin, Germany, 1991; p. 11. [Google Scholar]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar]
- Hay, P.J.; Wadt, W.R. Ab Initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Dunning, T.H.; Hay, P.J. Modern Theoretical Chemistry; Schaefer, H.F., III, Ed.; Plenum: New York, NY, USA, 1976; pp. 1–28. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Cances, M.T.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic Dielectrics. J. Chem. Phys. 1997, 107, 3032–3037. [Google Scholar] [CrossRef]
- Mennucci, B.; Tomasi, J. Continuum solvation models: A new approach to the problem of solute’s charge distribution and cavity boundaries. J. Chem. Phys. 1997, 106, 5151–5158. [Google Scholar] [CrossRef]
- Del Barrio, J.; Oriol, L.; Alcalá, R.; Sánchez, C. Azobenzene-Containing Linear-Dendritic Diblock Copolymers by Click Chemistry: Synthesis, Characterization, Morphological Study, and Photoinduction of Optical Anisotropy. Macromolecules 2009, 42, 5752–5760. [Google Scholar] [CrossRef]
- Iftime, G.; Lagugné-Labarthet, F.; Natansohn, A.; Rochon, P. Control of Chirality of an Azobenzene Liquid Crystalline Polymer with Circularly Polarized Light. J. Am. Chem. Soc. 2000, 122, 12646–12650. [Google Scholar] [CrossRef]
- Lagugne-labarthet, F.; Freiberg, S.; Pellerin, C.; Pezolet, M.; Natansohn, A.; Rochon, P. Spectroscopic and Optical Characterization of a Series of Azobenzene-Containing Side-Chain Liquid Crystalline Polymers. Macromolecules 2000, 33, 6815–6823. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ortíz-Palacios, J.; Rodríguez-Alba, E.; Avelar, M.; Martínez, A.; Carreón-Castro, M.D.P.; Rivera, E. Synthesis and Characterization of Novel Dendrons Bearing Amino-Nitro-Substituted Azobenzene Units and Oligo(ethylene glycol) Spacers: Thermal, Optical Properties, Langmuir Blodgett Films and Liquid-Crystalline Behaviour. Molecules 2013, 18, 1502-1527. https://doi.org/10.3390/molecules18021502
Ortíz-Palacios J, Rodríguez-Alba E, Avelar M, Martínez A, Carreón-Castro MDP, Rivera E. Synthesis and Characterization of Novel Dendrons Bearing Amino-Nitro-Substituted Azobenzene Units and Oligo(ethylene glycol) Spacers: Thermal, Optical Properties, Langmuir Blodgett Films and Liquid-Crystalline Behaviour. Molecules. 2013; 18(2):1502-1527. https://doi.org/10.3390/molecules18021502
Chicago/Turabian StyleOrtíz-Palacios, Jesús, Efraín Rodríguez-Alba, Mayra Avelar, Ana Martínez, Maria Del Pilar Carreón-Castro, and Ernesto Rivera. 2013. "Synthesis and Characterization of Novel Dendrons Bearing Amino-Nitro-Substituted Azobenzene Units and Oligo(ethylene glycol) Spacers: Thermal, Optical Properties, Langmuir Blodgett Films and Liquid-Crystalline Behaviour" Molecules 18, no. 2: 1502-1527. https://doi.org/10.3390/molecules18021502




