Synthesis of Thiophene and NO-Curcuminoids for Antiinflammatory and Anti-Cancer Activities
Abstract
:1. Introduction
2. Results and Discussion
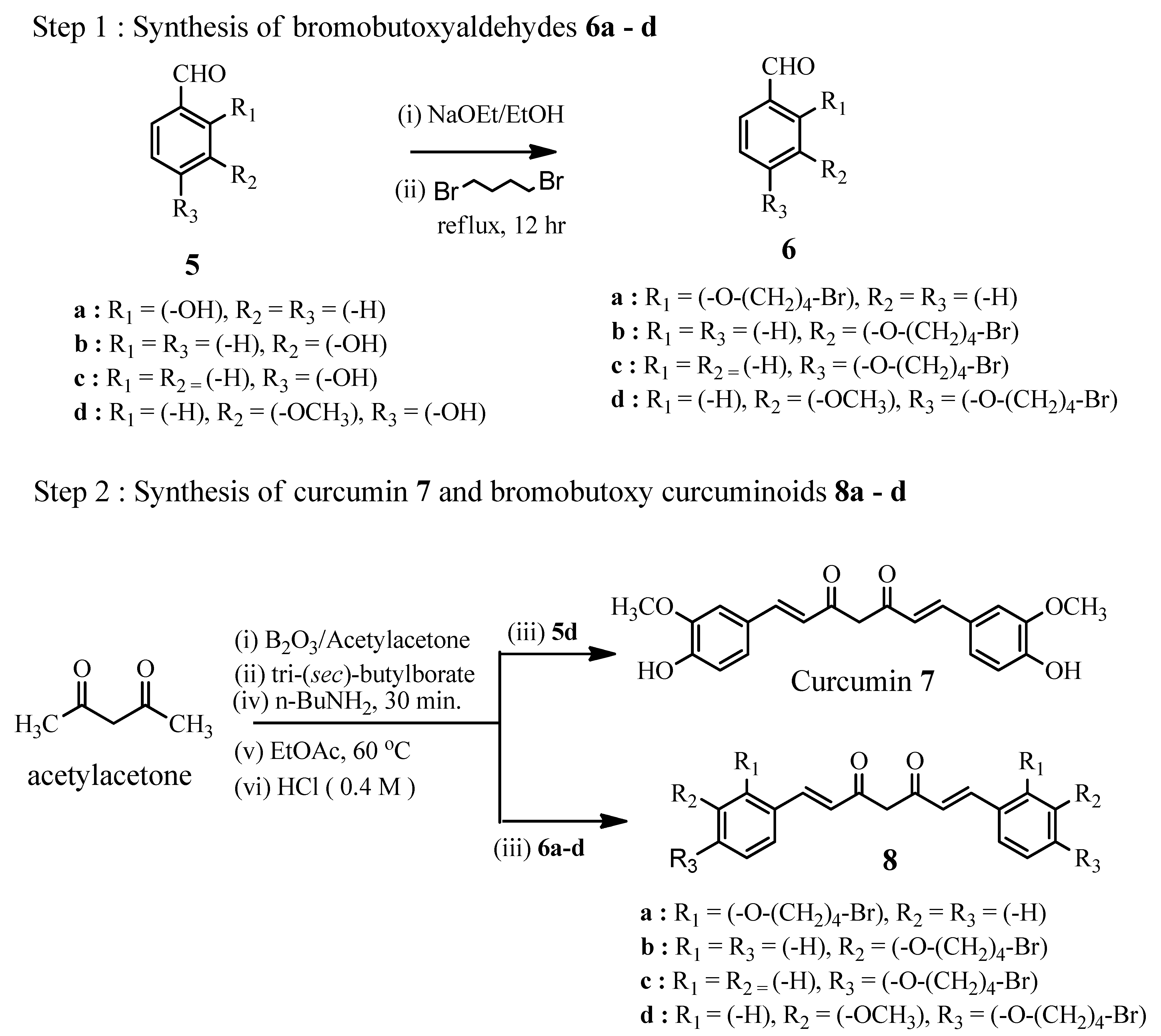
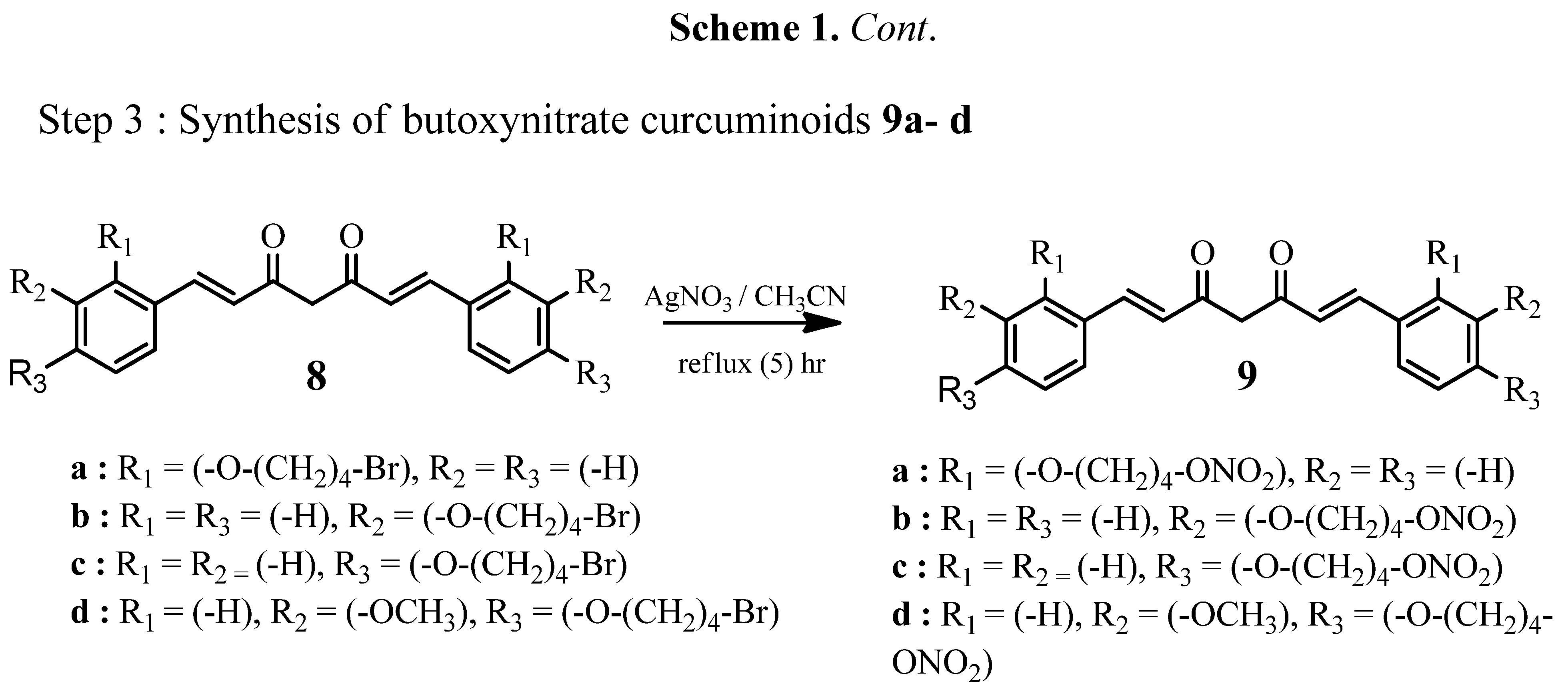
2.1. Chemistry
2.2. Pharmacology
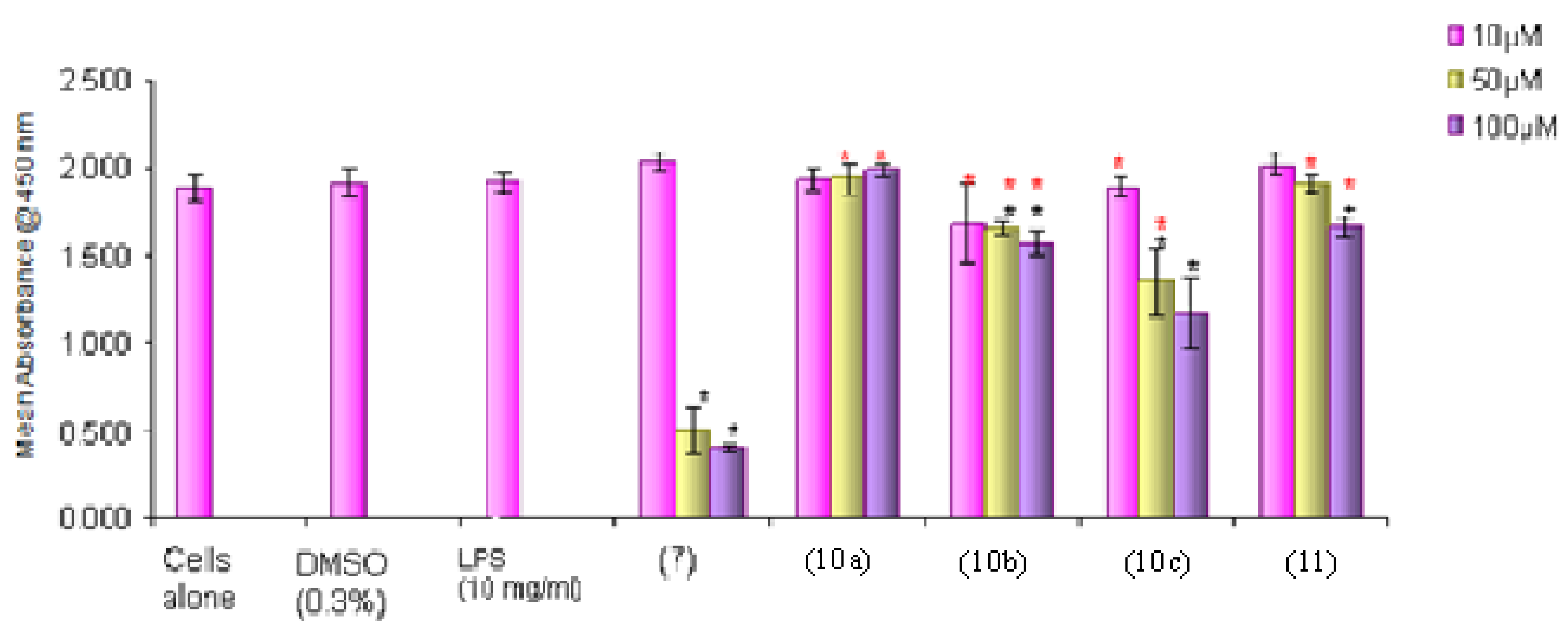
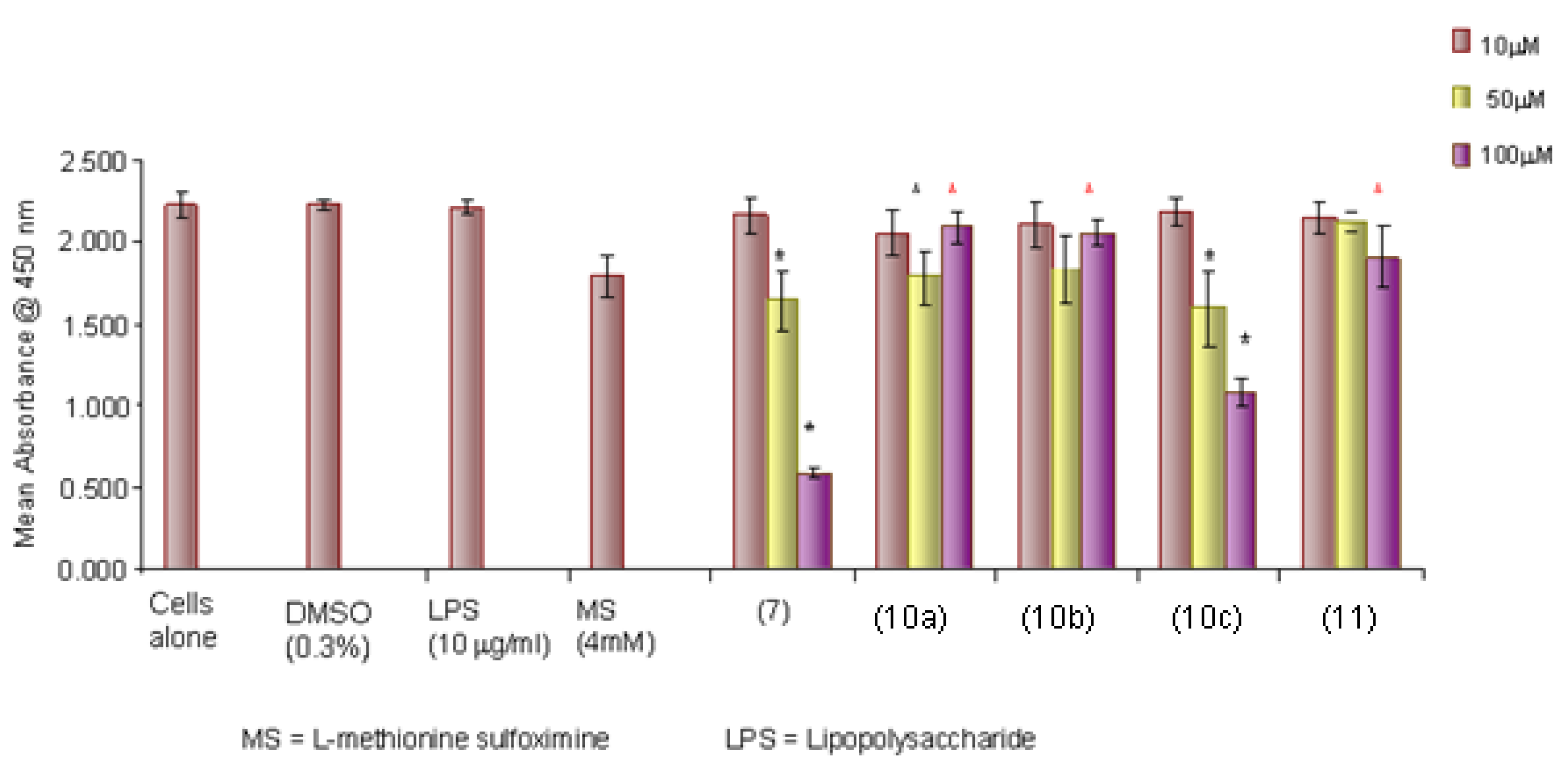
2.2.1. Cytotoxicity
2.2.2. Nitric Oxide Production
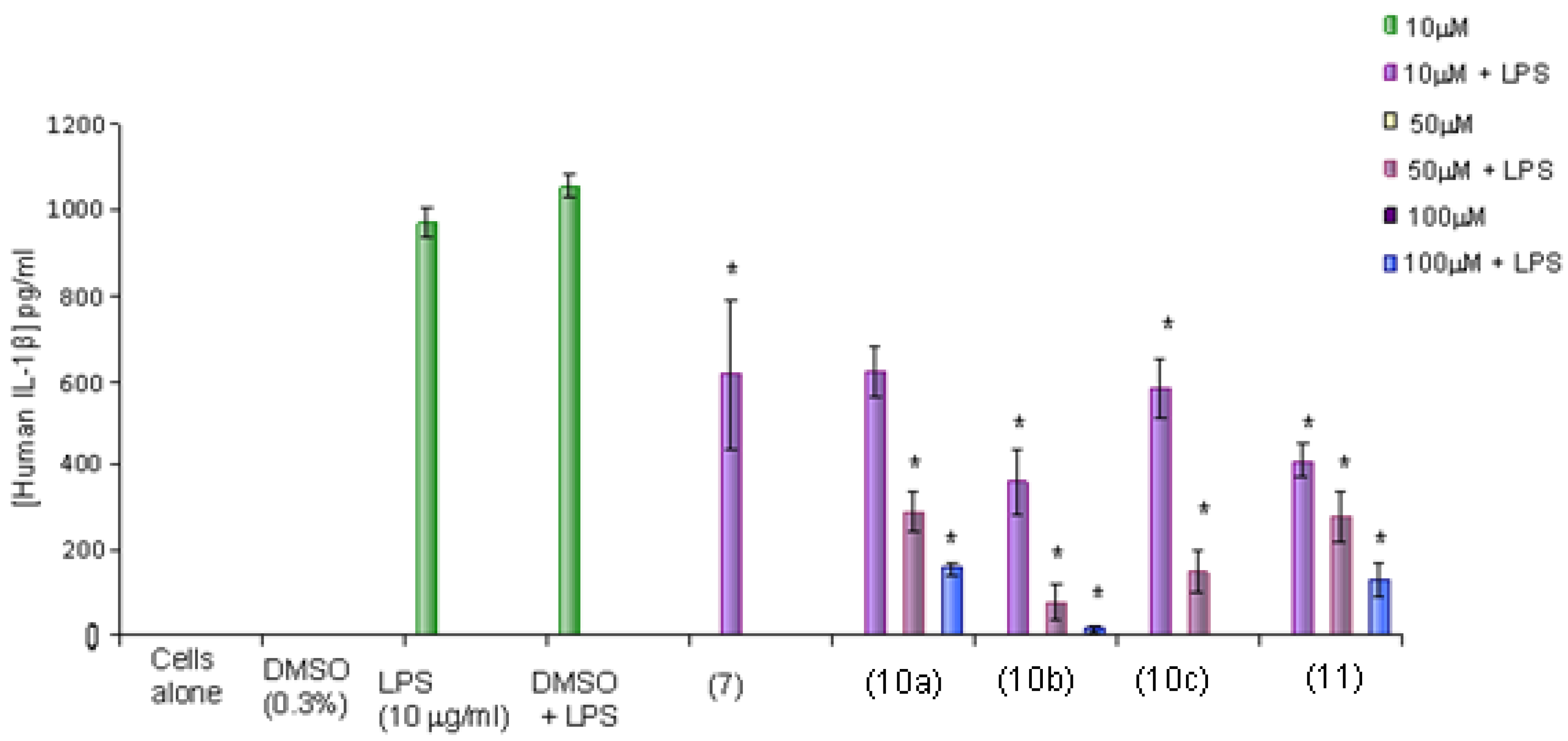
2.2.3. Cytokine Production
2.2.4. Chemokine Production
2.2.5. Discussion of Pharmacology and Conclusions
3. Experimental
3.1. Chemistry: General
3.1.1. Preparation of tri-sec-Butyl Borate
3.1.2. General Procedure for the Synthesis of Bromobutoxybenzaldehydes 6a–d
3.1.3. Synthesis of Curcuminoids
3.1.4. Synthesis of Nitroxybutyl Curcuminoids 9a–d
3.2. Pharmacology
3.2.1. General Procedures for Cell Cultures And Compound Preparations
3.2.2. Nitric Oxide Assay
3.2.3. Sandwich ELISA Assay
Acknowledgments
References
- Aggarwal, B.B.; Surh, Y.J.; Shishodia, S. (Eds.) The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: New York, NY, USA, 2007. [Google Scholar]
- Braun, L.; Cohen, N. Herbs and Natural Supplements: An. Evidence-Based Guide; Elsevier-Mosby: Sydney, Australia, 2004; pp. 358–368. [Google Scholar]
- Srimal, R.C.; Dhawan, B.N. Pharmacology of Diferuoyl Methane (Curcumin). A Non-steroidal Anti-inflammatory Agent. J. Pharm. Pharmacol. 1973, 25, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Nurfina, A.N.; Reksohadiprodja, M.S.; Timmerman, H.; Jenie, U.A.; Sugiyaanto, D.; van der Goot, H. Synthesis of some symmetrical curcumin derivatives and their antiinflammatory Activity. Eur. J. Med. Chem. 1997, 32, 321–3285. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Basu, N.; Ghatak, N.; Gujral, P.K. Antiinflammatory and Irritant Activities of Curcumin Analogs in Rats. Agents Actions 1982, 12, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.K.; Higo, T.; Hunter, W.L.; Burt, H.M. The antioxidants curcumin and quercetin Inhibit inflammatory processes associated with arthritis. Inflamm. Res. 2006, 55, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Sundaram, C.; Jhurani, S.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin and cancer: An “old age” desease with an “age old” solution. Cancer Lett. 2008, 267, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; El-Khatib, R.; Rainsford, K.D.; Whitehouse, M.W. Synthesis and anti-inflammatory properties of some aromatic and heterocyclic aromatic curcuminoids. Bioorg. Chem. 2012, 40, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Reuter, B.; Cicala, C.; McKnight, W.; Grisham, M.; Cirino, G. A diclofenac derivative without ulcerogenic properties. Eur. J. Pharmacol. 1994, 257, 249–255. [Google Scholar] [CrossRef]
- Arena, B.; Del Soldato, P. Nitric esters having a pharmacological activity and process for their preparation. US Patents 5,621,000, 15 April 1997. [Google Scholar]
- Del Sodato, P. Nitric esters having anti-inflammatory and/or analgesic activity and process for their preparation. US Patents 5,780,495, 14 July 1997. [Google Scholar]
- Chiroli, V.; Benedini, F.; Ongini, E.; Del Soldato, P. Nitric oxide-donating non-steroidal anti-inflammatory drugs: the case of nitroderivatives of aspirin. Eur. J. Med. Chem. 2003, 38, 441–446. [Google Scholar] [CrossRef]
- Fiorucci, S.; Antonelli, E. Dual COX-inhibitors: the answer is NO. Curr. Top. Med. Chem. 2005, 5, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Bolla, M.; Almirante, N.; Benedini, F. therapeutic potential of nitrate esters of commonly used drugs. Curr. Top. Med. Chem. 2005, 5, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Rigas, B.; Kashfi, K. Nitric oxide donating NSAIDs as agents for cancer prevention. Trends Mol. Med. 2004, 10, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.M.; Roseth, A.G.; Appleyard, C.B.; McKnight, W.; Del Soldato, P.; Calignano, A.; Cirino, G.; Wallace, J.L. NO-naproxen vs naproxen: Ulcerogenic, Alalgesic and anti-inflammatory effects. Aliment. Pharmacol. Ther. 1997, 11, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Nemmani, K.V.S.; Mali, S.V.; Borhade, N.; Pathan, A.R.; Karwa, M.; Pamidiboina, V.; Senthilkumar, S.P.; Gund, M.; Jain, A.K.; Mangu, N.K.; et al. NO-NSAIDs: Gastric-sparing nitric oxide-releasable prodrugs of non-steroidal anti-inflammatory drugs, Bioorg. Med. Chem. Lett. 2009, 19, 5297–5301. [Google Scholar] [CrossRef] [PubMed]
- Borhade, N.; Pathan, A.R.; Halder, S.; Karwa, M.; Dhiman, M.; Pamidiboina, V.; Gund, M.; Deshattiwar, J.J.; Mali, S.V.; Deshmukh, N.J.; et al. NO-NSAIDs. Part 3: Nitric Oxide-Releasing Prodrugs of Non-steroidal Anti-inflammatory Drugs. Chem. Pharm. Bull. 2012, 60, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Ranatunge, R.R.; Augustyniak, M.; Bandarage, U.K.; Cochran, E.D.; Earle, R.A.; Ellis, J.L.; GarveyY, D.S.; Janero, D.R.; Letts, L.G.; Martino, A.M.; et al. Synthesis and selective cycoloxygenase-2 inhibitory activity of a series of novel nitric oxide donor-containing pyrazoles. J. Med. Chem. 2004, 47, 2180–2193. [Google Scholar] [CrossRef] [PubMed]
- Viola, A.; Luster, A.D. Chemokines and their receptors: Drug targets in immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 171–197. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-Y.; Chu, L.-C.; Hua, K.-F.; Chao, L.-K. Heme oxygenase-1 mediates the anti-inflammatory effect of curcumin within LPS-stimulated human monocytes. J. Cell. Physiol. 2008, 215, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, V.; Schwalb, D.J.; Shumway, M.J.; Warren, M.C.; Wexler, R.S.; Zemtseva, I.S.; Zifcak, B.M.; Janero, D.R. Selective nitros(yl)ation induced in vivo by a nitric oxide-donating cyclooxygenase-2 inhibitor: A Nobonomic analysis. Free Radical Bio. Med. 2005, 39, 1191–1207. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. A clinical perspective of IL-1 beta as the gatekeeper of inflammation. Eur. J. Immunol. 2001, 41, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, K. Biological function of tumor necrosis factor cytokines and their receptors. Cytokine Growth F. R. 2003, 14, 185–191. [Google Scholar] [CrossRef]
- Abaza, M.S.; Khan, M.A.; Afzal, M. Chemistry, Biochemistry and Selective Cytotoxicity of Curcumin Analogues Against Human Cancer Cell Lines. In Curcumin: Biosynthesis, Medicinal Uses and Health Benefits; Sasaki, J., Kichida, M., Eds.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2012. [Google Scholar]
- Pabon, H.J.J. Synthesis of curcumin and related compounds. Recueil Des. Travaux Chimiques Des. Pays Bas 1964, 83, 379–386. [Google Scholar] [CrossRef]
- Huang, Y.; Li, N.; Liboni, K.; Neu, J. Glutamine decreases lipopolysaccharide-induced IL-8 production in CACO-2 cells through a non-NF-κB p50 mechanism. Cytokine 2003, 22, 77–83. [Google Scholar] [CrossRef]
- Rainsford, K.D.; Omar, H.; Ashraf, A.; Hewson, A.T.; Bunning, R.A.D.; Rishiraj, R.; Shepherd, P.; Seabrook, R.W. Recent pharmacokinetic and pharmacodynamic findings on oxaprozin. Inflammopharmacology 2002, 10, 185–239. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, Simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Correa, D.H.A.; Melo, P.S.; De Carvalho, C.A.A.; De Azevedo, M.B.M.; Duran, N.; Haun, M. Dehydrocrotonin and its β-cyclodextrin complex: Cytotoxicity in V79 fibroblasts and rat cultured hepatocytes. Eur. J. Pharm. 2005, 510, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Sastry, K.V.H.; Moudgal, R.P.; Mohan, J.; Tyagi, J.S.; Rao, G.S. Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal. Biochem. 2002, 306, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Hennig, C.; Rink, L.; Fagin, U.; Jabs, W.J.; Kirchner, H. The influence of naturally occurring heterophilic anti-immunoglobulin antibodies on direct measurement of serum proteins using sandwich ELISAs. J. Immunol. Methods 2000, 235, 71–80. [Google Scholar] [CrossRef]
- Eteshola, E.; Leckband, D. Development and characterization of an ELISA assay in PDMS microfluidic channels. Sensor. Actuat. B-Chem. 2001, 72, 129–133. [Google Scholar] [CrossRef]
- Nikulina, V.A.; Kizmi, E.A.; Massino, Y.S.; Segal, O.L.; Smirnova, M.B.; Avilov, V.V.; Saprigin, D.B.; Smotrov, S.P.; Tichtchenko, V.A.; Kolyaskina, G.I.; et al. Synergistic effects in antigen capture ELISA using three monoclonal antibodies directed at different epitopes of the same antigen. Clin. Chim. Acta 2000, 299, 25–44. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 9a–d, 10a–c and 11 are available from the authors. |







© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ahmed, M.M.; Khan, M.A.; Rainsford, K.D. Synthesis of Thiophene and NO-Curcuminoids for Antiinflammatory and Anti-Cancer Activities. Molecules 2013, 18, 1483-1501. https://doi.org/10.3390/molecules18021483
Ahmed MM, Khan MA, Rainsford KD. Synthesis of Thiophene and NO-Curcuminoids for Antiinflammatory and Anti-Cancer Activities. Molecules. 2013; 18(2):1483-1501. https://doi.org/10.3390/molecules18021483
Chicago/Turabian StyleAhmed, Mahera M., M. Akram Khan, and Kim Drummond Rainsford. 2013. "Synthesis of Thiophene and NO-Curcuminoids for Antiinflammatory and Anti-Cancer Activities" Molecules 18, no. 2: 1483-1501. https://doi.org/10.3390/molecules18021483




