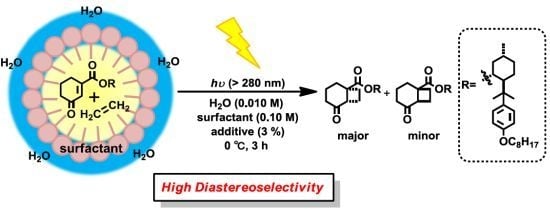
Diastereoselective [2+2] Photocycloaddition of Chiral Cyclic Enones with Olefins in Aqueous Media Using Surfactants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Substrates 3a, 3b

2.2. Photoreaction
2.2.1. Photoreaction of 3a, 3b with Ethylene Gas as Coupling Partner

| Entry | Substrate | Solvent | Surfactant | Additive | Conv./% b | Yield/% c | de/% b |
|---|---|---|---|---|---|---|---|
| (4+5) | (4-5)/(4+5) | ||||||
| 1 | 3a | H2O | − | − | 0 | d | d |
| 2 | H2O | SDS | − | 0 | d | d | |
| 3 | H2O | DAH | − | 0 | d | d | |
| 4 | H2O | SDS | CH2Cl2 | 100 | 83 | 23 | |
| 5 | H2O | SDS | toluene | 100 | 73 | 32 | |
| 6 | H2O | SDS | MCH e | 100 | 49 | 40 | |
| 7 | H2O | DAH | CH2Cl2 | 100 | 50 | 30 | |
| 8 | H2O | DAH | toluene | 100 | 61 | 35 | |
| 9 | H2O | DAH | MCH e | 100 | 41 | 40 | |
| 10 | CH2Cl2 | − | − | 100 | 83 | 19 | |
| 11 | toluene | − | − | 100 | 104 | 31 | |
| 12 | MCH e | − | − | 100 | 69 | 49 | |
| 13 | 3b | H2O | − | − | 0 | d | d |
| 14 | H2O | SDS | − | 0 | d | d | |
| 15 | H2O | DAH | − | 0 | d | d | |
| 16 | H2O | SDS | CH2Cl2 | 62 | 52 f | 52 | |
| 17 | H2O | SDS | toluene | 73 | 16 f | 42 | |
| 18 | H2O | SDS | MCH e | 92 | 64 f | 37 | |
| 19 | H2O | DAH | CH2Cl2 | 62 | 52 f | 52 | |
| 20 | H2O | DAH | toluene | 73 | 16 f | 42 | |
| 21 | H2O | DAH | MCH e | 92 | 64 f | 37 | |
| 22 | CH2Cl2 | − | − | 100 | 98 | 12 | |
| 23 | toluene | − | − | 100 | 99 | 23 | |
| 24 | MCH e | − | − | 100 | 81 | 41 |
2.2.2. Photoreaction of 3a,b with Cyclopentene (liquid) as Coupling Partner

| Entry | Substrate | Solvent | Surfactant | Additive | Conv./% b | Yield/% c | Ratio | de (%) d | |
|---|---|---|---|---|---|---|---|---|---|
| (syn:anti) d | |||||||||
| (6-9) | (6:(8+9)) | syn 6 | anti (8-9)/(8+9) | ||||||
| 1 | 3a | H2O | − | − | 0 | e | e | e | e |
| 2 | H2O | SDS | − | 100 | 87 | 39:61 | >99 | 11 | |
| 3 | H2O | DAH | − | 100 | 76 | 51:49 | >99 | 11 | |
| 4 | H2O | SDS | CH2Cl2 | 100 | 66 | 56:44 | >99 | 65 | |
| 5 | H2O | SDS | toluene | 100 | 80 | 55:45 | >99 | 60 | |
| 6 | H2O | SDS | MCH | 100 | 63 | 59:41 | >99 | 49 | |
| 7 | H2O | DAH | CH2Cl2 | 100 | 32 | 52:48 | >99 | 53 | |
| 8 | H2O | DAH | toluene | 100 | 40 | 49:51 | >99 | 55 | |
| 9 | H2O | DAH | MCH | 100 | 32 | 47:53 | >99 | 54 | |
| 10 | CH2Cl2 | − | − | 100 | 88 | 53:47 | >99 | 59 | |
| 11 | toluene | − | − | 100 | 101 | 51:49 | >99 | 86 | |
| 12 | MCH | − | − | 100 | 83 | 52:48 | >99 | 53 | |
| 13 | 3b | H2O | − | − | 0 | e | e | e | e |
| 14 | H2O | SDS | − | 100 | 47 | 43:57 | >99 | 52 | |
| 15 | H2O | DAH | − | 100 | 45 | 45:55 | >99 | 54 | |
| 16 | H2O | SDS | CH2Cl2 | 100 | 28 f | 43:57 | >99 | 52 | |
| 17 | H2O | SDS | toluene | 100 | 48 f | 41:59 | >99 | 53 | |
| 18 | H2O | SDS | MCH | 100 | 66 f | 43:57 | >99 | 52 | |
| 19 | H2O | DAH | CH2Cl2 | 100 | 44 f | 40:60 | >99 | 43 | |
| 20 | H2O | DAH | toluene | 100 | 49 f | 40:60 | >99 | 47 | |
| 21 | H2O | DAH | MCH | 100 | 31 f | 42:58 | >99 | 37 | |
| 22 | CH2Cl2 | − | − | 100 | 77 | 40:60 | >99 | 55 | |
| 23 | toluene | − | − | 100 | 99 | 40:60 | >99 | 53 | |
| 24 | MCH | − | − | 100 | 81 | 40:60 | >99 | 53 | |
3. Experimental
3.1. General
3.2. Materials
3.2.1. Preparation of (1R,2S,5R)-5-methyl-2-(2-(4-(octyloxy)phenyl)propan-2-yl)cyclohexanol (1b)
3.2.2. Preparation of (1R,2S,5R)-5-methyl-2-(2-(4-(octyloxy)phenyl)propan-2-yl)cyclohexyl 3-oxocyclohex-1-enecarboxylate (3b)
3.2.3. Photoproduct; (1S,6S)-(1R,2S,5R)-5-methyl-2-(2-(4-(octyloxy)phenyl)propan-2-yl)cyclohexyl 5-oxobicyclo[4.2.0]octane-1-carboxylate (4b, Major Compound)
3.2.4. Photoproduct; (1R,6R)-(1R,2S,5R)-5-methyl-2-(2-(4-(octyloxy)phenyl)propan-2-yl)cyclohexyl 5-oxobicyclo[4.2.0]octane-1-carboxylate (5b, Minor Compound)
3.2.5. Photoproduct; (3aR,3bS,7aS,7bS)-(1R,2S,5R)-5-methyl-2-(2-(4-(octyloxy)phenyl)propan-2-yl)cyclohexyl 7-oxodecahydro-1H-cyclopenta[3,4]cyclobuta[1,2]benzene-3b-carboxylate (6b, cis-syn-cis, Major Compound)
3.2.6. Photoproduct; (3aS,3bS,7aS,7bR)-(1R,2S,5R)-5-methyl-2-(2-(4-(octyloxy)phenyl)propan-2-yl)cyclohexyl 7-oxodecahydro-1H-cyclopenta[3,4]cyclobuta[1,2]benzene-3b-carboxylate (8b, cis-anti-cis, Major Compound)
3.2.7. Photoproduct; (3aR,3bR,7aR,7bS)-(1R,2S,5R)-5-methyl-2-(2-(4-(octyloxy)phenyl)propan-2-yl)cyclohexyl 7-oxodecahydro-1H-cyclopenta[3,4]cyclobuta[1,2]benzene-3b-carboxylate (9b, cis-anti-cis, Minor Compound)
3.3. Photolysis with Additive and Surfactant
3.3.1. Photolysis of 3a,b with Ethylene
3.3.2. Photolysis of 3a,b with Cyclopentene
4. Conclusions
Acknowledgments
References
- Tundo, P.; Anastas, P.; Black, D.S.; Breen, J.; Collins, T.; Memoli, S.; Miyamoto, J.; Polyakoff, M.; Tumas, W. Synthetic pathways and processes in green chemistry. Introductory overview. Pure Appl. Chem. 2000, 72, 1207–1228. [Google Scholar] [CrossRef]
- Anastas, P.T.; Kirchhoff, M.M. Origins, current status, and future challenges of green chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef]
- Beach, E.S.; Cui, Z.; Anastas, P.T. Green chemistry: A design framework for sustainability. Energy Environ. Sci. 2009, 2, 1038–1049. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Chanda, A.; Fokin, V.V. Organic synthesis “on water”. Chem. Rev. 2009, 109, 725–748. [Google Scholar] [CrossRef]
- Butler, R.N.; Coyne, A.G. Water: Nature’s reaction enforcer-comparative effects for organic synthesis “in-water” and “on-water”. Chem. Rev. 2010, 110, 6302–6337. [Google Scholar] [CrossRef]
- Oelgemoeller, M.; Healy, N.; de Oliveira, L.; Jung, C.; Mattay, J. Green photochemistry: solar-chemical synthesis of juglone with medium concentrated sunlight. Green Chem. 2006, 8, 831–834. [Google Scholar] [CrossRef]
- Protti, S.; Fagnoni, M. The sunny side of chemistry: Green synthesis by solar light. Photochem. Photobiol. Sci. 2009, 8, 1499–1516. [Google Scholar] [CrossRef]
- Iriondo-Alberdi, J.; Greaney, M.F. Photocycloaddition in natural product synthesis. Eur. J. Org. Chem. 2007, 4801–4815. [Google Scholar] [CrossRef]
- Bach, T.; Hehn, J.P. Photochemical reactions as key steps in natural product synthesis. Angew. Chem. Int. Ed. 2011, 50, 1000–1045. [Google Scholar] [CrossRef]
- Furutani, A.; Katayama, K.; Uesima, Y.; Ogura, M.; Tobe, Y.; Kurosawa, H.; Tsutsumi, K.; Morimoto, T.; Kakiuchi, K. Asymmetric [2+2] photocycloaddition of cycloalkenone-cyclodextrin complexes to ethylene. Chirality 2006, 18, 217–221. [Google Scholar] [CrossRef]
- Tsutsumi, K.; Endou, K.; Furutani, A.; Ikki, T.; Nakano, H.; Shintani, T.; Morimoto, T.; Kakiuchi, K. Diastereoselective [2+2] photocycloaddition of chiral cyclohexenonecarboxylates to ethylene. Chirality 2003, 15, 504–509. [Google Scholar] [CrossRef]
- Furutani, A.; Tsutsumi, K.; Nakano, H.; Morimoto, T.; Kakiuchi, K. Highly diastereoselective synthesis of bicyclo[4.2.0]octanone derivatives by the [2+2] photocycloaddition of chiral cyclohexenonecarboxylates to ethylene. Tetrahedron Lett. 2004, 45, 7621–7624. [Google Scholar] [CrossRef]
- Tsutsumi, K.; Nakano, H.; Furutani, A.; Endou, K.; Merpuge, A.; Shintani, T.; Morimoto, T.; Kakiuchi, K. Novel enhancement of diastereoselectivity of [2+2] photocycloaddition of chiral cyclohexenones to ethylene by adding naphthalenes. J. Org. Chem. 2004, 69, 785–789. [Google Scholar] [CrossRef]
- Tsutsumi, K.; Terao, K.; Yamaguchi, H.; Yoshimura, S.; Morimoto, T.; Kakiuchi, K.; Fukuyama, T.; Ryu, I. Diastereoselective [2+2] photocycloaddition of chiral cyclic enone and cyclopentene using a microflow reactor system. Chem. Lett. 2010, 39, 828–829. [Google Scholar] [CrossRef]
- Inhülsen, I.; Akiyama, N.; Tsutsumi, K.; Nishiyama, Y.; Kakiuchi, K. Highly diastereodifferentiating and regioselective [2+2]-photoreactions using methoxyaromatic menthyl cyclohexenone carboxylates. Tetrahedron 2013, 69, 782–790. [Google Scholar] [CrossRef]
- Coyle, E.E.; Joyce, K.; Nolan, K.; Oelgemoeller, M. Green photochemistry: The use of microemulsions as green media in photooxygenation reactions. Green Chem. 2010, 12, 1544–1547. [Google Scholar] [CrossRef]
- Drillaud, N.; Banaszak-Leonard, E.; Pezron, I.; Len, C. Synthesis and evaluation of a photochromic surfactant for organic reactions in aqueous media. J. Org. Chem. 2012, 77, 9553–9561. [Google Scholar] [CrossRef]
- Tsutsumi, K.; Yanagisawa, Y.; Furutani, A.; Morimoto, T.; Kakiuchi, K.; Wada, T.; Mori, T.; Inoue, Y. Diastereodifferentiating the [2+2] photocycloaddition of ethylene to arylmenthyl cyclohexenonecarboxylates: Stacking-driven enhancement of the product diastereoselectivity that is correlated with the reactant ellipticity. Chem. Eur. J. 2010, 16, 7448–7455. [Google Scholar] [CrossRef]
- Corey, E.J.; Ensley, H.E. Preparation of an optically active prostaglandin intermediate via asymmetric induction. J. Am. Chem. Soc. 1975, 97, 6908–6909. [Google Scholar] [CrossRef]
- Yang, D.; Xu, M.; Bian, M. Chiral auxiliaries for asymmetric radical cyclization reactions: Application to the enantioselective synthesis of (+)-triptocallol. Org. Lett. 2001, 3, 111–114. [Google Scholar] [CrossRef]
- Shintani, T.; Kusabiraki, K.; Hattori, A.; Furutani, A.; Tsutsumi, K.; Morimoto, T.; Kakiuchi, K. Diastereoselective [2+2] photocycloaddition of polymer-supported cyclic chiral enone with ethylene. Tetrahedron Lett. 2004, 45, 1849–1851. [Google Scholar] [CrossRef]
- Terao, K.; Nishiyama, Y.; Aida, S.; Tanimoto, H.; Morimoto, T.; Kakiuchi, K. Diastereodifferentiating [2+2] photocycloaddition of chiral cyclohexenone carboxylates with cyclopentene by a microreactor. J. Photochem. Photobiol. A 2012, 242, 13–19. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nishiyama, Y.; Shibata, M.; Ishii, T.; Morimoto, T.; Tanimoto, H.; Tsutsumi, K.; Kakiuchi, K. Diastereoselective [2+2] Photocycloaddition of Chiral Cyclic Enones with Olefins in Aqueous Media Using Surfactants. Molecules 2013, 18, 1626-1637. https://doi.org/10.3390/molecules18021626
Nishiyama Y, Shibata M, Ishii T, Morimoto T, Tanimoto H, Tsutsumi K, Kakiuchi K. Diastereoselective [2+2] Photocycloaddition of Chiral Cyclic Enones with Olefins in Aqueous Media Using Surfactants. Molecules. 2013; 18(2):1626-1637. https://doi.org/10.3390/molecules18021626
Chicago/Turabian StyleNishiyama, Yasuhiro, Mikiko Shibata, Takuya Ishii, Tsumoru Morimoto, Hiroki Tanimoto, Ken Tsutsumi, and Kiyomi Kakiuchi. 2013. "Diastereoselective [2+2] Photocycloaddition of Chiral Cyclic Enones with Olefins in Aqueous Media Using Surfactants" Molecules 18, no. 2: 1626-1637. https://doi.org/10.3390/molecules18021626