Structural Requirements for the Antifungal Activities of Natural Drimane Sesquiterpenes and Analogues, Supported by Conformational and Electronic Studies
Abstract
:1. Introduction

2. Results and Discussion
2.1. Chemistry
2.1.1. Compounds Obtained from 1
2.1.2. Compounds Obtained from 2

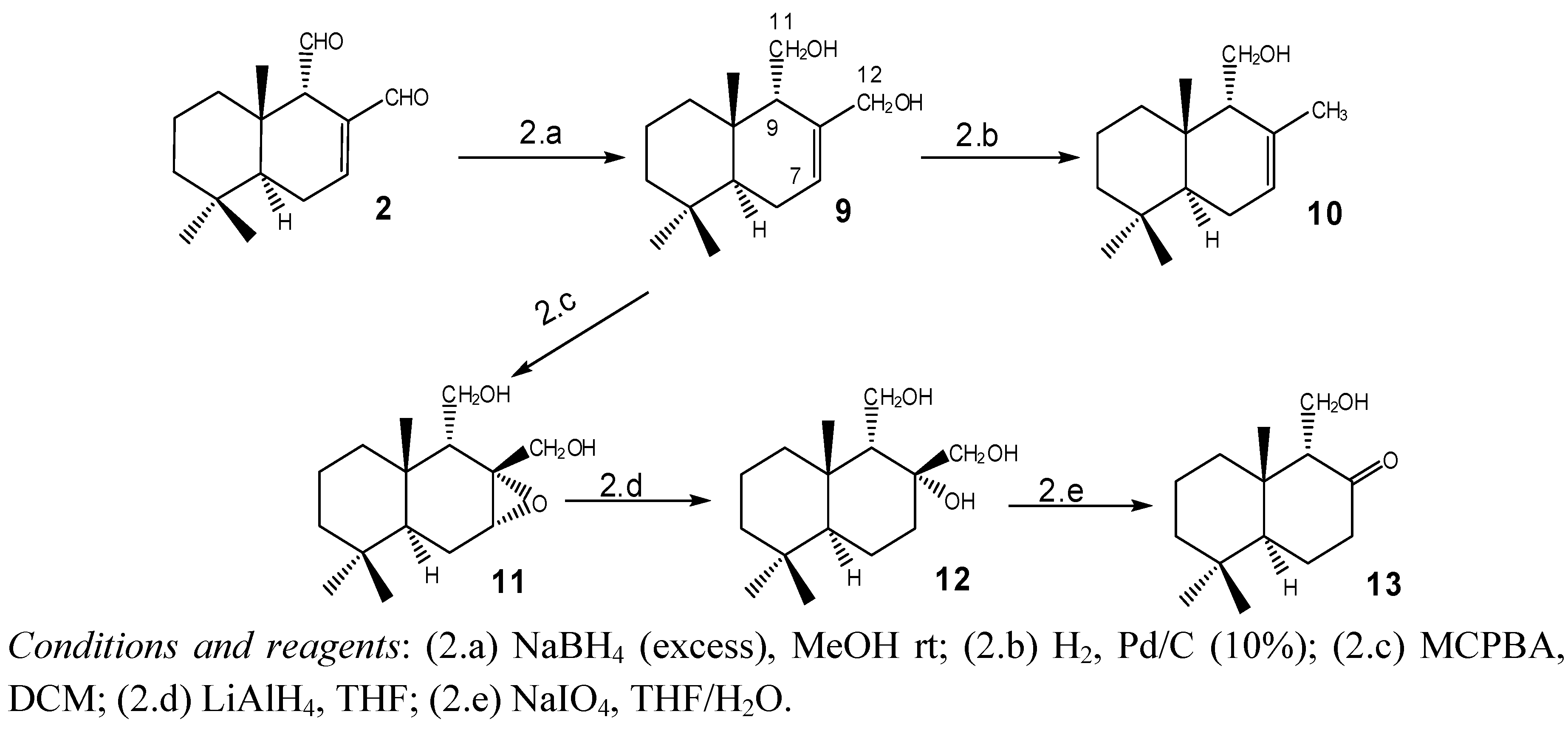
2.1.3. Compounds Obtained from 3
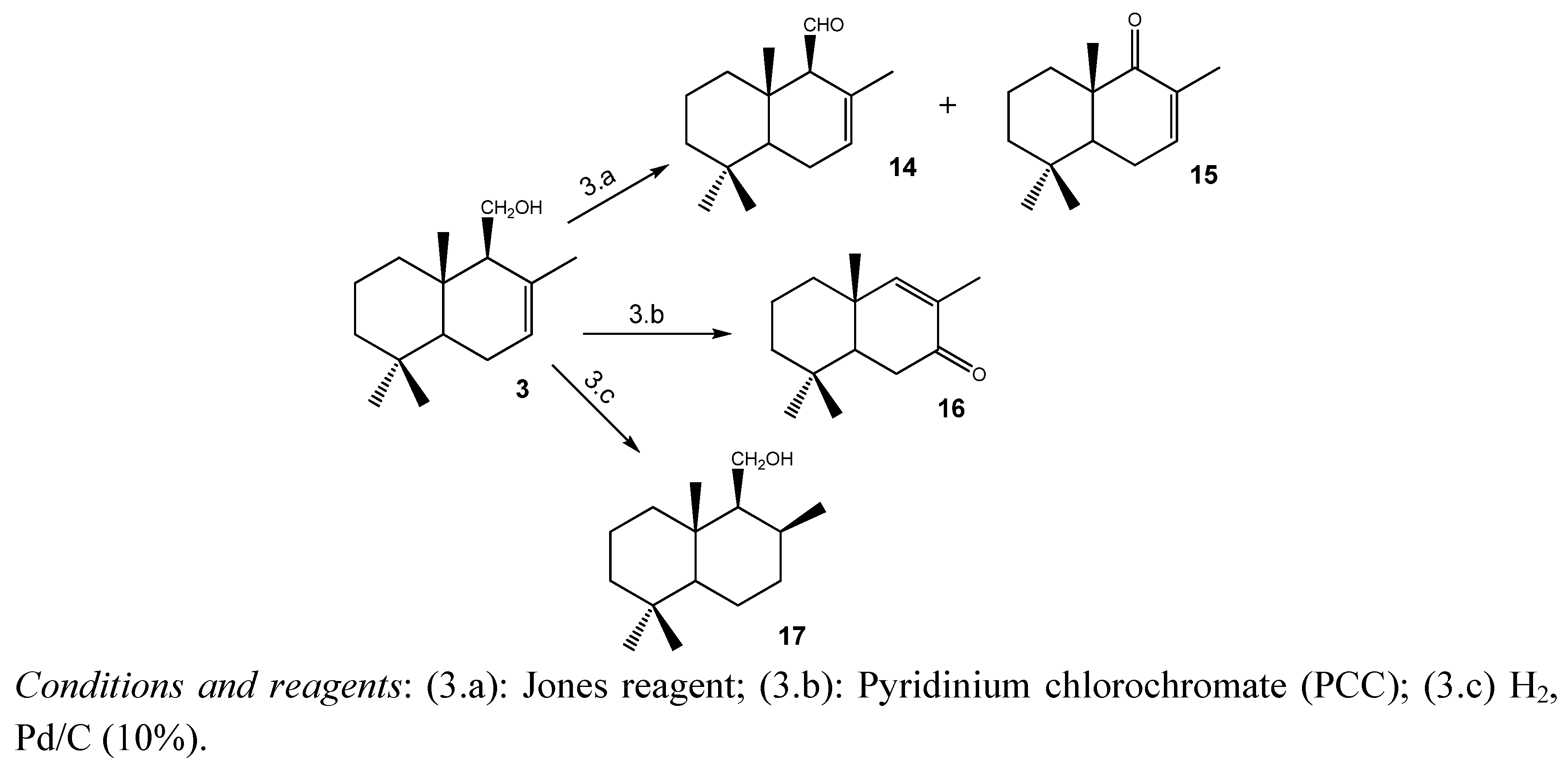
2.2. Antifungal Activity
| Compounds | Yeasts | Aspergillus spp | Dermatophytes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nº | Structure | Ca | Sc | Cn | Afum | Afl | An | Mg | Tr | Tm | ||
| 1 |  | MIC100 | 3.9 | 15.6 | 7.8 | I | I | I | 62.5 | 62.5 | 62.5 | |
| MFC | 7.8 | 31.2 | 7.8 | - | - | - | 125 | 125 | 125 | |||
| 2 | 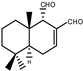 | |||||||||||
| MIC100 | 62.5 | 62.5 | 62.5 | I | I | I | 62.5 | 62.5 | 62.5 | |||
| MFC | 125 | 125 | 125 | - | - | - | 125 | 125 | 125 | |||
| 3 | 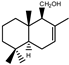 | |||||||||||
| MIC100 | I | I | 125 | I | I | I | 62.5 | 62.5 | 62.5 | |||
| MFC | - | - | I | - | - | - | 125 | 125 | 125 | |||
| 4 | 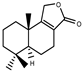 | |||||||||||
| MIC100 | I | 250 | I | I | I | I | 125 | 62.5 | 62.5 | |||
| MFC | - | - | - | - | - | - | 250 | 125 | 125 | |||
| 5 | 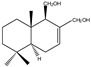 | |||||||||||
| MIC100 | 125 | 250 | 250 | I | I | I | 250 | 250 | 250 | |||
| MFC | - | - | - | - | - | - | 250 | 250 | 250 | |||
| 6 | 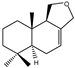 | |||||||||||
| MIC100 | 250 | 250 | 125 | I | I | I | 62.5 | 62.5 | 62.5 | |||
| MFC | I | I | 250 | - | - | - | 125 | 125 | 125 | |||
| 7 | 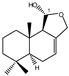 | |||||||||||
| MIC100 | 250 | 250 | 125 | I | I | I | 31.2 | 31.2 | 31.2 | |||
| MFC | I | I | 250 | - | - | - | 125 | 125 | 125 | |||
| 8 |  | |||||||||||
| MIC100 | 250 | 250 | 125 | I | I | I | 62.5 | 62.5 | 62.5 | |||
| MFC | I | I | 250 | - | - | - | 125 | 125 | 125 | |||
| 9 | 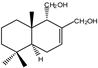 | |||||||||||
| MIC100 | 250 | 250 | 125 | I | I | I | 125 | 125 | 250 | |||
| MFC | I | I | 250 | - | - | - | 125 | 250 | 250 | |||
| 10 | 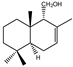 | |||||||||||
| MIC100 | 125 | 125 | 31.2 | I | I | I | 125 | 62.5 | 250 | |||
| MFC | 250 | 125 | 62.5 | - | - | - | 250 | 125 | 250 | |||
| 11 | 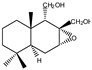 | |||||||||||
| MIC100 | I | I | I | I | I | I | I | I | I | |||
| MFC | - | - | - | - | - | - | - | - | - | |||
| 12 | 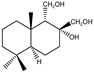 | MIC100 | I | I | I | I | I | I | I | I | I | |
| MFC | - | - | - | - | - | - | - | - | - | |||
| 13 | 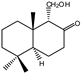 | MIC100 | I | I | I | I | I | I | 250 | 250 | 250 | |
| MFC | - | - | - | - | - | - | I | 250 | 250 | |||
| 14 | 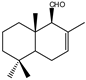 | MIC100 | 125 | 125 | 31.2 | I | I | I | 62.5 | 62.5 | 125 | |
| MFC | 125 | 125 | 31.2 | - | - | - | 125 | 125 | 250 | |||
| 15 | 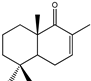 | MIC100 | I | I | 250 | I | I | I | 250 | 250 | 250 | |
| MFC | - | - | - | - | - | - | 250 | 250 | 250 | |||
| 16 | 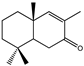 | MIC100 | 250 | 250 | 250 | I | I | I | 250 | 250 | 250 | |
| MFC | I | I | 250 | - | - | - | 250 | 250 | 250 | |||
| 17 | 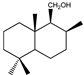 | MIC100 | I | I | I | I | I | I | I | I | I | |
| MFC | - | - | - | - | - | - | - | - | - | |||
| St drugs Amph B Terbinafine Ketoconazole | ||||||||||||
| MIC | 0.78 | 0.50 | 0.25 | 0.50 | 0.50 | 0.50 | 0.12 | 0.075 | 0.075 | |||
| MIC | 1.56 | 3.12 | 0.39 | 0.78 | 0.78 | 1.56 | 0.04 | 0.01 | 0.025 | |||
| MIC | 0.5 | 0.5 | 0.25 | 0.12 | 0.5 | 0.25 | 0.05 | 0.025 | 0.025 | |||
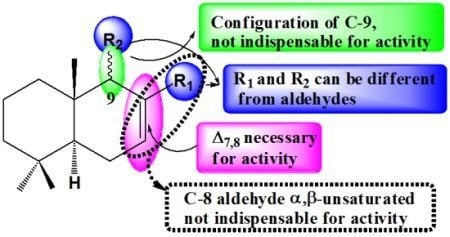
2.3. Structure-Activity Relationships (SAR)
| MIC50 (µg/mL) | |||
|---|---|---|---|
| Ca | Cn | LogP | |
| 1 | 1.9 | 1.9 | 2.33 |
| 2 | 31.2 | 31.2 | 2.33 |
| 3 | 62.5 | 31.2 | 3.83 |
| 4 | 62.5 | 62.5 | 3.35 |
| 5 | 250 | 250 | 2.76 |
| 6 | 62.5 | 31.2 | 3.40 |
| 7 | 62.5 | 31.2 | 3.24 |
| 8 | 31.2 | 31.2 | 3.67 |
| 9 | 250 | 31.2 | 2.77 |
| 10 | 62.5 | 15.6 | 3.83 |
| 14 | 62.5 | 62.5 | 3.53 |
| 15 | 250 | I | 3.84 |
| 16 | 31.2 | 62.5 | 3.37 |
| 17 | I | I | 4.30 |
| Amphotericin B | 0.78 | 0.25 | |
2.3.1. Role of the Δ7,8-Double Bond in the Drimane Skeleton Alone and in α,β Position Respective to the Aldehyde on C-8
2.3.2. Role of the Configuration of C-9
2.3.3. Role of an Aldehyde at C-9 and of a CH2OH at C-8
2.4. Conformational and Electronic Studies
2.4.1. Conformational Analysis
- (a)
- The conformational behavior of torsional angles ф1-ф5 which determines the spatial ordering of ring A.
- (b)
- The orientation of torsional angles ф6-ф9 which determines the overall shape of ring B. In this ring, they are also important torsional angles ф10 and ф11, which are related to CH2OH substituents as R2 or R1.




3. Experimental
3.1. Chemistry
3.1.1. Isolation of Natural Compounds 1–4
3.1.2. General Procedure for Synthesis of 5 and 6
3.1.3. Synthesis of (−)-(1R,5aS,9aS,9bR)-6,6,9a-Trimethyl-1,3,5,5a,6,7,8,9,9a,9b-decahydro naphto[2,1c]furan-1-ol (isodrimeninol, 7)
3.1.4. Synthesis of (−)-(1R,4aS,8aS)-5,5,8a-Trimethyl-2-vinyl-1,4,4a,5,6,7,8,8a-octahydro naphtalene-1-carbaldehyde (8) [11]
3.1.5. Synthesis of (+)-(5S,10S)-(9S)-7-Drimene-11,12diol (isodrimendiol, 9)
3.1.6. Synthesis of (+) [(1R,4aS,8aS)-2,5,5,8a-Tetramethyl-1,4,4a,5,6,7,8,8a-octahydro naphthalen-1-yl]methanol (isodrimenol, 10)
3.1.7. Synthesis of (−)-7α,8-Epoxy-(9S)-drimane-11,12-diol (11) [20]
3.1.8. Synthesis of (−)-(9S)-Drimane-8,11,12-triol (12) [20]
3.1.9. Synthesis of (−)-11-Hydroxy-(9R)-12-nordriman-8-one (13) [20]
3.1.10. General Procedure for Synthesis of 14 and 15 [13,16]
3.1.11. Synthesis of (−)-(4aS,8aS)-3,4a,8,8-Tetramethyl-4a,5,6,7,8,8a-hexahydro-1H-naphtalen-2-one (16) [14,17]
3.1.12. Synthesis of (+) [(1S,2S,8aS)-2,5,5,8a-Tetramethyldecahydronaphthalen-1-yl]methanol (drimanol, 17)
3.2. Antifungal Evaluation
3.2.1. Microorganisms and Media
3.2.2. Antifungal Susceptibility Testing
3.3. Calculations Methods
4. Conclusions
Acknowledgments
- Samples Availability: Samples of the compounds 1–4 are available from the authors.
References
- Del Vitto, L.; Petenatti, E.; Petenatti, M. Recursos herbolarios de San Luis (Argentina) 2a parte: Plantas exóticas cultivadas, adventicias y/o naturalizadas. Multequina 1998, 7, 29–48. [Google Scholar]
- Derita, M.; Leiva, M.; Zacchino, S. Influence of plant part, season of collection and content of the main active constituent, on the antifungal properties of Polygonum acuminatum Kunt. J. Ethnopharmacol. 2009, 124, 377–383. [Google Scholar] [CrossRef]
- Mc Callion, R.; Cole, A.; Walker, J.; Blunt, J.; Munro, M. Antibiotic substances from New Zealand plants. Planta Med. 1982, 44, 134–138. [Google Scholar] [CrossRef]
- De Almeida Alves, T.; Ribeiro, F.; Kloos, H.; Zani, C. Polygodial, the fungitoxic component from the Brazilian medicinal plant Polygonum punctatum. Mem. Inst. Oswaldo Cruz. 2001, 96, 831–833. [Google Scholar] [CrossRef]
- Taniguchi, M.; Adachi, T.; Oi, S.; Kimura, A.; Katsumura, S.; Isoe, S.; Kubo, I. Structure-activity relationship of the Warburgia sesquiterpene dialdehydes. Agric. Biol. Chem. 1984, 48, 73–78. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Lunde, C.; Kubo, I. In vitro antifungal susceptibilities ofCandida albicans and other fungal pathogens to polygodial, a sesquiterpene dialdehyde. Planta Med. 1998, 65, 204–208. [Google Scholar]
- Fujita, K.; Kubo, I. Multifunctional action of antifungal polygodial against Saccharomyces cerevisiae: Involvement of pyrrole formation on cell surface in antifungal action. Bioorg. Med. Chem. 2005, 13, 6742–6747. [Google Scholar] [CrossRef]
- Anke, H.; Sterner, O. Comparison of the antimicrobial and cytotoxic activities of twenty unsaturated sesquiterpene dialdehydes from plants and mushrooms. Planta Med. 1991, 57, 344–347. [Google Scholar] [CrossRef]
- Derita, M.; Zacchino, S. Validation of the ethnopharmacological use of Polygonum persicaria for its antifungal properties. Nat. Prod. Commun. 2011, 6, 931–933. [Google Scholar]
- Taniguchi, M.; Yano, Y.; Tada, E.; Ikenishi, K.; Oi, S.; Haraguchi, H.; Hashimoti, K.; Kubo, I. Mode of action of polygodial, an antifungal sesquiterpene dialdehyde. Agric. Biol. Chem. 1988, 52, 1409–1414. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI), Reference Method for Broth Dilution Antifungal Susceptibility Testing for Yeasts (M27 A3), 3rd ed; CLSI: Wayne, PA, USA,, 2008; Volume 28, No. 14, pp. 1–25.
- Clinical and Laboratory Standards Institute (CLSI), Reference Method for Broth Dilution Antifungal Susceptibility Testing for and for Filamentous Fungi (M38 A2), 2nd ed; CLSI: Wayne, PA, USA, 2008; Volume 28, No. 16, pp. 1–35.
- Cuellar, M.; Salas, C.; Cortés, M.; Morello, A.; Maya, J.; Preite, M. Synthesis and in vitro trypanocide activity of several drimane-quinone derivatives. Bioorg. Med. Chem. 2003, 11, 2489–2497. [Google Scholar] [CrossRef]
- Cuellar, M.; Moreno, L.; Preite, M. Regioselective oxidative fragmentation of drimanic terpene alcohols: a short, easy and efficient access to natural and synthetic 11-nordrimane terpene derivatives. ARKIVOC 2003, 10, 169–177. [Google Scholar]
- Sakio, Y.; Hirano, Y.; Hayashi, M.; Komiyama, K.; Ishibashi, M. Dendocarbins A-N, new drimane sesquiterpenes from the nudibranch Dendrodoris carbunculosa. J. Nat. Prod. 2001, 64, 726–731. [Google Scholar] [CrossRef]
- Matsuda, H.; Pongpiriyadacha, Y.; Morikawa, T.; Kashima, Y.; Nakano, K.; Yoshikawa, M. Protective effects of polygodial and related compounds on ethanol-induced gastric mucosal lesions in rats: Structural requirements and mode of action. Bioorg. Med. Chem. Lett. 2002, 12, 477–482. [Google Scholar] [CrossRef]
- Cuellar Fritis, M. Synthesis of sesquiterpenquinones and aza-anthraquinones derivatives using polygodial as chiral synthon. Pontificia Universidad Católica de Chile, Santiago, Chile, 2002; p. 155. [Google Scholar]
- Feng, J.; Yang, C.; Zhang, D.; Wang, J.; Fu, H.; Chen, H.; Li, X. Catalytic transfer hydrogenolysis of α-methylbenzyl alcohol using palladium catalysts and formic acid. Appl. Catal. A Gen. 2009, 354, 38–43. [Google Scholar] [CrossRef]
- Donelly, D.; O´Reilly, J.; Chiaroni, A.; Polonsky, J. Crystal structure and absolute configurationof a new sesquiterpenoid metabolite of Fomes annosus, 7α,8β,11-trihydroxydrimane. Chem. Soc. Perkin Trans. 1 1980, 2196–2199. [Google Scholar]
- González Sierra, M.; Colombo, M.; Zudenigo, M.; Rúveda, E. 13C NMR spectral analysis of grindelane diterpenoid acids. Phytochemistry 1984, 23, 1685–1689. [Google Scholar] [CrossRef]
- Cortés, M.; Moreno, L.; López, J. Partial Synthesis of (−)-11,12-dinordriman-8-one and the (−)-enantiomer of polywood. J. Chem. Res. (S) 1998, 36–37. [Google Scholar]
- Scher, J.; Speakman, J.; Zapp, J.; Becker, H. Bioactivity guided isolation of antifungal compounds from the liverwort Bazzania trilobata (L.) S. F. Gray. Phytochemistry 2004, 65, 2583–2588. [Google Scholar]
- Moreno-Osorio, L.; Espinoza, L.; Cuellar, M.; Preite, M. LTA-mediated synthesis and complete assignment of 1H and 13C NMR data of two natural 11-nordrimanes: Isonordrimenone and polygonone. Magn. Reson. Chem. 2007, 45, 993–996. [Google Scholar] [CrossRef]
- Hellou, J.; Andersen, R. Terpenoids from the dorid nudibranch Cadlina luteomarginata. Tetrahedron 1982, 38, 1875–1879. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, K.; Zhang, Y.; Huang, R.; Bian, J.; Zheng, Ch.; Sun, H.; Chen, Z.; Sun, N.; An, R.; et al. High-throughput synergy screening identifies microbial metabolites as combination agents for the treatment of fungal infections. Proc. Natl. Acad. Sci. USA 2007, 104, 4606–4011. [Google Scholar]
- Singh, N. Impact of current transplantation practices on the changing epidemiology of infections in transplant recipients. Lancet Infect. Dis. 2003, 3, 156–161. [Google Scholar] [CrossRef]
- Pfaller, M.; Diekema, D. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef]
- Barrero, A.; Oltra, J.E.; Alvarez, M.; Raslan, D.; Saúde, D.; Akssira, M. New sources and antifungal activity of sesquiterpene lactones. Fitoterapia 2000, 71, 60–64. [Google Scholar] [CrossRef]
- Justicia, J.; Álvarez de Cienfuegos, L.; Estévez, R.; Paradas, M.; Lasanta, A.; Oller, J.; Rosales, A.; Cuerva, J.; Oltra, E. Ti-catalyzed transannular cyclization of epoxygermacrolides. Synthesis of antifungal (+)-tuberiferine and (+)-dehydrobrachylaenolide. Tetrahedron 2008, 64, 11938–11943. [Google Scholar]
- Vargas, L.; Castelli, M.V.; Kouznetsov, V.; Urbina, J.; López, S.; Sortino, M.; Enriz, R.; Ribas, J.C.; Zacchino, S. In vitro antifungal activity of new series of homoallylamines and related compounds with inhibitory properties of the synthesis of fungal cell wall polymers. Bioorg. Med. Chem. 2003, 11, 1531–1550. [Google Scholar] [CrossRef]
- Musiol, R.; Jampilek, J.; Buchta, V.; Silva, L.; Niedbala, H.; Podeszwa, B.; Palka, A.; Majerz-Maniecka, K.; Oleksyn, B.; Polanski, J. Antifungal properties of new series of quinoline derivatives. Bioorg. Med. Chem. 2006, 14, 3592–3598. [Google Scholar] [CrossRef]
- Setiadi, D.; Chass, G.; Torday, L.; Varro, A.; Papp, J. Vitamin E models. Conformational analysis and stereochemistry of tetralin, chroman, thiochroman and selenochroman. J. Mol. Struct. (Theochem.) 2002, 594, 161–172. [Google Scholar]
- Suvire, F.; Andreu, I.; Bermejo, A.; Zamora, M.; Cortés, D.; Enriz, R. Conformational study of N-alkyl-benzyltetrahydroisoquinolines alkaloid. J. Mol. Struct. (Theochem.) 2003, 666, 109–116. [Google Scholar]
- Villagra, S.; Bernini, M.; Rodrıguez, A.; Zacchino, S.; Kouznetsov, V.; Enriz, R. Conformational and electronic study of homoallylamines with inhibitory properties against polymers of fungal cell wall. J. Mol. Struct. (Theochem.) 2003, 666, 587–598. [Google Scholar]
- Politzer, P.; Truhlar, D. Chemical Applications of Atomic and Molecular Electrostatic Potentials; Plenum Publishing: New York, NY, USA, 1981. [Google Scholar]
- Carrupt, P.; El Tayar, N.; Karlén, A.; Festa, B. Molecular electrostatic potentials for characterizing drug-biosystem interactions. Methods Enzymol. 1991, 202, 638–650. [Google Scholar]
- Greeling, P.; Langenaeker, W.; De Proft, F.; Baeten, A. Molecular Electrostatic Potentials: Concepts and Applications. Theoretical and Computational Chemistry; Elsevier Science B.V.: Amsterdam, The Netherlands, 1996; p. 587. [Google Scholar]
- Voda, K.; Boh, B.; Vrtacnik, M. A quantitative structure-antifungal activity relationship study of oxygenated aromatic essential oil compounds using data structuring and PLS regression analysis. J. Mol. Model. 2004, 10, 76–84. [Google Scholar] [CrossRef]
- Leal, P.; Mascarello, A.; Derita, M.; Zuljan, F.; Nunes, R.; Zacchino, S.; Yunes, R. Relation between lipophylicity of alkyl gallates and antifungal activity against yeasts and filamentous fungi. Bioorg. Med. Chem. Lett. 2009, 19, 1793–1796. [Google Scholar]
- Meléndez Gómez, C.; Kouznetsov, V.; Sortino, M.; Alvarez, S.; Zacchino, S. In vitro antifungal activity of polyfunctionalized 2-(hetero)arylquinolines prepared through imino Diels Alder reactions. Bioorg. Med. Chem. 2008, 16, 7908–8920. [Google Scholar] [CrossRef]
- Barfknecht, C.; Nichols, D. Correlation of psychotomimetic activity of phenetylamines and amphetamines with 1-octanol-water partition coefficients. J. Med. Chem. 1975, 18, 208–210. [Google Scholar] [CrossRef]
- López, S.; Castelli, M.; Zacchino, S.; Domínguez, J.; Lobo, G.; Charris-Charris, J.; Cortés, J.; Ribas, J.; Devia, C.; Rodríguez, A.; Enriz, R. In vitro antifungal evaluation and structure-activity relationships of a new series of chalcone derivatives and synthetic analogues, with inhibitory properties against polymers of the fungal cell wall. Bioorg. Med. Chem. 2001, 9, 1999–2013. [Google Scholar] [CrossRef]
- Jansen, B.; de Groot, A. Occurrence, biological activity and synthesis of drimane sesquiterpenoids. Nat. Prod. Rep. 2004, 21, 449–477. [Google Scholar] [CrossRef]
- Appel, H.; Bond, R.; Overton, K. The constitution and stereochemistry of valdiviolide, fuegin, winterin and futronolide. Tetrahedron 1963, 19, 635–641. [Google Scholar] [CrossRef]
- Ying, B.; Peiser, G.; Ji, Y.; Mathias, K.; Tutko, D.; Hwang, Y. Phytotoxic sesquiterpenoids from Canella winterana. Phytochemistry 1995, 38, 909–915. [Google Scholar]
- Barnes, C.; Loder, J. The structure of polygodial: A new sesquiterpene dialdehyde from Polygonum hydropiper L. Austr. J. Chem. 1962, 15, 322–327. [Google Scholar] [CrossRef]
- Guillerm, D.; Delarue, M.; Jalali–Naini, M.; Lemaître, P.; Lallemand, J-Y. Synthesis of all possible stereoisomers of polygodial. Tetrahedron Lett. 1984, 25, 1043–1048. [Google Scholar] [CrossRef]
- Castelli, V.; Lodeyro, A.; Malheiros, A.; Zacchino, S.; Roveri, O. Inhibition of the mitochondrial ATP synthesis by polygodial, a naturally occurring dialdehyde unsaturated sesquiterpene. Biochem. Pharmacol. 2005, 70, 82–89. [Google Scholar]
- Malheiros, A.; Cechinel Filho, V.; Schmitt, C.; Yunes, R.; Escalante, A.; Svetaz, L.; Zacchino, S.; Delle Monache, F. Antifungal activity of drimane sesquiterpenes from Drimys brasiliensis using bioassay-guided fractionation. J. Pharm. Pharm. Sci. 2005, 8, 335–339. [Google Scholar]
- Rodriguez, B.; Zapata, N.; Medina, P.; Viñuela, E. A complete 1H and 13C NMR data assignment for four drimane sesquiterpenoids isolated from Drimys winterii. Magn. Reson. Chem. 2005, 43, 82–84. [Google Scholar] [CrossRef]
- Urban, S.; Capon, R. Absolute stereochemistry of puupehenone and related metabolites. J. Nat. Prod. 1996, 59, 900–901. [Google Scholar] [CrossRef]
- Hueso-Rodriguez, J.; Rodríguez, B. A new and efficient route to optically active drimanes. Synthesis of (+)-winterin, (+)-confertifolin, (+)-isodrimenin and (+)-bicyclofarnesol. Tetrahedron 1989, 45, 1567–1576. [Google Scholar]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Montgomery, J., Jr.; Vreven, T.; Kudin, K.; Burant, J.; et al. Gaussian 03, Revision B.05; Gaussian, Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, M. Results obtained with the correlation energy density functionals of Becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–207. [Google Scholar] [CrossRef]
- Becke, A. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Becke, A. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Santagata, L.; Suvire, F.; Enriz, R.; Torday, L.; Csizmadia, I. A geometrical algorithm to search the conformational space of flexible molecules. J. Mol. Struct. (Theochem.) 1999, 465, 33–67. [Google Scholar]
- Santagata, L.; Suvire, F.; Enriz, R. An analytic ring closure condition for geometrical algorithm to search the conformational space. J. Mol. Struct. (Theochem.) 2000, 507, 89–95. [Google Scholar]
- Santagata, L.; Suvire, F.; Enriz, R. Partially relaxed ring closure conditions for geometrical algorithm to search the conformational space for minimum energy conformations. J. Mol. Struct. (Theochem.) 2001, 536, 173–188. [Google Scholar]
- Politzer, P.; Daiker, K. The Force Concept in Chemistry; Deb, M.B., Ed.; van Nostrand Reinhold: New York, NY, USA, 1981. [Google Scholar]
- Flükiger, P.; Lüthi, H.; Portmann, S.; Weber, J. MOLEKEL 4.0; Swiss Center for Scientific Computing: Manno, Switzerland, 2000. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Derita, M.; Montenegro, I.; Garibotto, F.; Enriz, R.D.; Fritis, M.C.; Zacchino, S.A. Structural Requirements for the Antifungal Activities of Natural Drimane Sesquiterpenes and Analogues, Supported by Conformational and Electronic Studies. Molecules 2013, 18, 2029-2051. https://doi.org/10.3390/molecules18022029
Derita M, Montenegro I, Garibotto F, Enriz RD, Fritis MC, Zacchino SA. Structural Requirements for the Antifungal Activities of Natural Drimane Sesquiterpenes and Analogues, Supported by Conformational and Electronic Studies. Molecules. 2013; 18(2):2029-2051. https://doi.org/10.3390/molecules18022029
Chicago/Turabian StyleDerita, Marcos, Iván Montenegro, Francisco Garibotto, Ricardo D. Enriz, Mauricio Cuellar Fritis, and Susana A. Zacchino. 2013. "Structural Requirements for the Antifungal Activities of Natural Drimane Sesquiterpenes and Analogues, Supported by Conformational and Electronic Studies" Molecules 18, no. 2: 2029-2051. https://doi.org/10.3390/molecules18022029