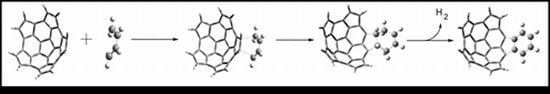
Growth of Fullerene Fragments Using the Diels-Alder Cycloaddition Reaction: First Step towards a C60 Synthesis by Dimerization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Frontier Molecular Orbital Analysis
2.2. Kinetic and Thermodynamic Analysis
3. Experimental
4. Conclusions
Acknowledgments
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Bukminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Krätschmer, W.; Fostiropoulos, K.; Huffman, D.R. The infrared and ultraviolet absorption spectra of laboratory-produced carbon dust: Evidence of the presence of the C60 molecule. Chem. Phys. Lett. 1990, 170, 167–170. [Google Scholar] [CrossRef]
- Scott, L.T.; Boorum, M.M.; McMahon, B.J.; Hagen, S.; Mack, J.; Blank, J.; Wegner, H.; de Meijere, A. A rational synthesis of C60. Science 2002, 295, 1500–1503. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.T.; Petrukhina, M.A. Preface. In Fragments of Fullerenes and Carbon Nanotubes: Designed Synthesis, Unusual Reactions and Coordination Chemistry; Scott, L.T., Petrukhina, M.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. vii–x. [Google Scholar]
- Krätschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A new form of carbon. Nature 1990, 347, 354–358. [Google Scholar] [CrossRef]
- Otero, G.; Biddau, G.; Sánchez-Sánchez, C.; Caillard, R.; López, M.F.; Rogero, C.; Palomares, F.J.; Cabello, N.; Basanta, M.A.; Ortega, J.; et al. Fullerenes from aromatic precursors by surface-catalysed cyclodehydrogenation. Nature 2008, 454, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.T. Methods for the chemical synthesis of fullerenes. Angew. Chem. Int. Ed. 2004, 43, 4994–5007. [Google Scholar] [CrossRef] [PubMed]
- Tsefrikas, V.M.; Scott, L.T. Geodesic polyarenes by flash vacuum pyrolysis. Chem. Rev. 2006, 106, 4868–4884. [Google Scholar] [CrossRef] [PubMed]
- Geneste, F.; Moradpour, A.; Dive, G.; Peeters, D.; Malthête, J.; Sadoc, J.-F. Retrosynthetic analysis of fullerene C60: Structure, stereochemistry, and calculated stability of C30 fragments. J. Org. Chem. 2002, 67, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Abdourazak, A.H.; Marcinow, Z.; Sygula, A.; Sygula, R.; Rabideau, P.W. Buckybowls 2. Toward the total synthesis of Bukminsterfullerene (C60): Benz[5,6]-as-indaceno-[3,2,1,8,7-mnopqr]indeno[4,3,2,1-cdef]chrysene. J. Am. Chem. Soc. 1995, 117, 6410–6411. [Google Scholar] [CrossRef]
- Hagen, S.; Bratcher, M.S.; Erickson, M.S.; Zimmermann, G.; Scott, L.T. Novel syntheses of three C30H12 bowl-shaped polycyclic aromatic hydrocarbons. Angew. Chem. Int. Ed. 1997, 36, 406–408. [Google Scholar] [CrossRef]
- Anet, F.A.L.; Muira, S.S.; Siegel, J.; Mislow, K. La Coupe du Roi and its relevance to stereochemistry. Combination of two homochiral molecules to give an achiral product. J. Am. Chem. Soc. 1983, 105, 1419–1926. [Google Scholar] [CrossRef]
- Scott, L.T. Polycyclic aromatic hydrocarbon bowls, baskets, balls and tubes: Challenging targets for chemical synthesis. Polycyclic Aromat. Compd. 2010, 30, 247–259. [Google Scholar] [CrossRef]
- Fort, E.H.; Donovan, P.M.; Scott, L.T. Diels-Alder reactivity of polycyclic aromatic hydrocarbon bay regions: Implications for metal-free growth of single-chirality carbon nanotubes. J. Am. Chem. Soc. 2009, 131, 16006–16007. [Google Scholar] [CrossRef] [PubMed]
- Fort, E.H.; Scott, L.T. Gas-phase Diels-Alder cycloaddition of benzyne to an aromatic hydrocarbon bay region: Groundwork for the selective solvent-free growth of armchair carbon nanotubes. Tetrahedron Lett. 2011, 52, 2051–2053. [Google Scholar] [CrossRef]
- Fukui, K.; Yonezawa, T.; Shingu, H. A molecular-orbital theory of reactivity in aromatic hydrocarbons. J. Chem. Phys. 1952, 20, 722–725. [Google Scholar] [CrossRef]
- Fukui, K.; Yonezawa, T.; Nagata, C.; Shingu, H. Molecular-orbital theory of orientation in aromatic, heteroaromatic, and other conjugated molecules. J. Chem. Phys. 1954, 22, 1433–1442. [Google Scholar] [CrossRef]
- Flemig, I. Molecular Orbitals and Organic Chemical Reactions; John Wiley & Sons Ltd: London, UK, 2009; pp. 224–242. [Google Scholar]
- Park, Y.S.; Lee, B.-S.; Lee, I. Density functional theory studies of hetero-Diels-Alder reactions. New J. Chem. 1999, 27, 707–715. [Google Scholar] [CrossRef]
- Hehre, W.J.; Radom, L.; Schleyer, P.v.R.; Pople, J.A. Ab Initio Molecular Orbital Theory; Wiley: New York, NY, USA, 1986; Chapter 4. [Google Scholar]
- Mendoza, J.A.; García-Pérez, E.; Jiménez-Vázquez, H.A.; Tamariz, J. Effect of aryl substituents on the reactivity of the captodative olefins 1-acetylvinyl arenecarboxylates. J. Mex. Chem. Soc. 2006, 50, 47–56. [Google Scholar]
- Houk, K.N.; Lin, Y.-T.; Brown, F.K. Evidence for the concerted mechanism of the Diels-Alder reaction of butadiene with ethylene. J. Am. Chem. Soc. 1986, 108, 554–556. [Google Scholar] [CrossRef] [PubMed]
- Houk, K.N.; Loncharich, R.J.; Blake, J.F.; Jorgensen, W.L. Substituent effects and transition structures for Diels-Alder reactions of butadiene and cyclopentadiene with cyanoalkenes. J. Am. Chem. Soc. 1989, 111, 9172–9176. [Google Scholar] [CrossRef]
- Guner, V.; Khuong, K.S.; Leach, A.G.; Lee, P.S.; Bartberger, M.D.; Houk, K.N. A standard set of pericyclic reactions of hydrocarbons for the benchmarking of computational methods: The performance of ab initio, Density functional, CASSCF, CASPT2, and CBS-QB3 methods for the prediction of activation barriers, Reaction energetics, and transition state geometries. J. Phys. Chem. A 2003, 107, 11445–11459. [Google Scholar]
- Ishida, K.; Morokuma, K.; Komornic, A. The intrinsic reaction coordinate. An ab inito calculation for HNC→HCN and H-+CH4→CH4+H-. J. Chem. Phys. 1977, 66, 2153–2156. [Google Scholar] [CrossRef]
- Fukui, K. The paths of chemical reaction—The IRC approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. An improved algorithm for reaction path following. J. Chem. Phys. 1989, 90, 2154–2161. [Google Scholar] [CrossRef]
- Kistiakowsky, G.B.; Ruhoff, J.R.; Smith, H.A.; Vaughan, W.E. Heats of organic reactions. IV. Hydrogenation of some dienes and of benzene. J. Am. Chem. Soc. 1936, 58, 146–153. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A., Jr.; Stratmann, R.E.; Burant, J.C.; et al. GAUSSIAN 03 (Revision A.9); Gaussian, Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Lee, K.H.; Lee, C.; Kang, J.; Park, S.S.; Lee, J.; Lee, S.K.; Bohme, D.K. Preferential site of attack on fullerene cations: Frontier orbitals and rate coefficients. J. Phys. Chem. A 2006, 110, 11730–11733. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.I.; Moncada, J.L.; Larenas, J.M. The dual descriptor to measure local reactivity on Buckminster fullerenes: An analysis within the framework of conceptual DFT. J. Mol. Model. 2010, 16, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Petrukhina, M.A.; Andreini, K.W.; Mack, J.; Scott, L.T. X-ray quality geometries of geodesic polyarenes from theoretical calculations: What levels of theory are reliable? J. Org. Chem. 2005, 70, 5713–5716. [Google Scholar] [CrossRef] [PubMed]
- Osuna, S.; Morera, J.; Cases, M.; Morokuma, K.; Solà, M. Diels-Alder reaction between ciclopentadiene and C60: An analysis of the performance of the ONIOM method for the study of chemical reactivity in fullerenes and nanotubes. J. Phys. Chem. A 2009, 113, 9721–9726. [Google Scholar] [CrossRef] [PubMed]
- Ikuma, N.; Susami, Y.; Oshima, T. Kinetics and regioselectivity in the Diels-Alder reaction of fulleroids vs. methanofullerene and C60. Org. Biomol. Chem. 2010, 8, 1394–1398. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Moreno, G.V.; González-Zamora, E.; Méndez, F. Oxazole as an electron-deficient diene in the Diels-Alder reaction. Org. Lett. 2011, 13, 6358–6361. [Google Scholar] [CrossRef] [PubMed]
- Damoun, S.; Van de Woude, G.; Méndez, F.; Geerlings, P. Local softness as a regioselectivity indicator in [4+2] cycloaddition reactions. J. Phys. Chem. A 1997, 101, 886–893. [Google Scholar] [CrossRef]
Sample Availability: Not available. |







| Molecule | EHOMO | ELUMO |
|---|---|---|
| Ethylene | −10.11 (−10.07) a | 4.88 (4.87) a |
| Butadiene | −8.77 (−8.82) a | 3.45 (3.59) a |
| 1 | −7.28 | 1.38 |
| 2 | −7.18 | 0.67 |
| Reaction | ΔENEDDA | ΔEIEDDA | δΔE |
|---|---|---|---|
| e + b | 13.66 (13.69) a | 13.56 (13.66) a | −0.09 |
| 1 + e | 12.17 | 11.49 | −0.67 |
| 2 + e | 12.06 | 10.78 | −1.27 |
| 1 + b | 10.15 | 10.73 | 0.58 |
| 2 + b | 9.45 | 10.63 | 1.18 |
| TS | r1 | r2 | Δr |
|---|---|---|---|
| e + b | 2.27 (2.21) a | 2.27 (2.21) a | 0 |
| 1 + e concave | 2.09 | 2.07 | 0.02 |
| 2 + e concave | 2.07 | 2.06 | 0.01 |
| 1 + e convex | 2.41 | 1.68 | 0.73 |
| 2 + e convex | 2.31 | 1.63 | 0.67 |
| 1 + b concave | 2.49 | 1.92 | 0.58 |
| 2 + b concave | 2.28 | 2.25 | 0.03 |
| 1 + b convex | 2.36 | 2.02 | 0.34 |
| 2 + b convex | 2.27 | 2.27 | 0 |
| Reaction | Ea (kcal mol−1) | ΔGa (kcal mol−1) | ΔHa (kcal mol−1) | TΔSa (kcal mol−1) |
|---|---|---|---|---|
| e + b | 24.8 (27.5) a (24.2–26.7)b (25.9) c | 36.35 | 23.45 (24.2)d (24.9)e | −12.91 (−13.32)f (−12.76)g |
| 1 + e concave | 40.71 | 52.88 | 39.62 | −13.25 |
| 2 + e concave | 45.63 | 57.21 | 44.70 | −12.51 |
| 1 + e convex | 67.89 | 80.22 | 66.75 | −13.47 |
| 2 + e convex | 73.54 | 85.84 | 72.35 | −13.49 |
| 1 + b concave | 39.01 | 51.86 | 38.36 | −13.49 |
| 2 + b concave | 31.05 | 43.53 | 30.43 | −13.10 |
| 1 + b convex | 33.11 | 46.03 | 32.47 | −13.56 |
| 2 + b convex | 22.61 | 35.52 | 22.02 | −13.51 |
| Reaction | ΔGr (kcal mol−1) | ΔHr (kcal mol−1) | TΔSr (kcal mol−1) |
|---|---|---|---|
| e + b | −23.76 | −37.82 (−37.9) a (−36.6) b | −14.06 (−14.1)c |
| 1 + e concave | 16.03 | 1.73 | −14.29 |
| 2 + e concave | 21.90 | 7.86 | −14.04 |
| 1 + e convex | 49.53 | 35.36 | −14.17 |
| 2 + e convex | 60.84 | 46.82 | −14.02 |
| 1 + b concave | 6.62 | −8.49 | −15.11 |
| 2 + b concave | −10.41 | −25.50 | −15.09 |
| 1 + b convex | 10.34 | −4.28 | −14.62 |
| 2 + b convex | −13.05 | −27.88 | −14.82 |
| System | ΔGr(kcal mol−1) | ΔHr (kcal mol−1) | TΔSr (kcal mol−1) |
|---|---|---|---|
| e + b | 0.09 | 17.20 (21.13) a | 17.12 |
| 1 + e concave | −11.34 | 5.93 | 17.27 |
| 2 + e concave | −12.23 | 4.96 | 17.19 |
| 1 + e convex | −44.85 | −27.70 | 17.15 |
| 2 + e convex | −51.16 | −33.99 | 17.17 |
| 1 + b concave | −22.84 | −5.58 | 17.26 |
| 2 + b concave | −13.12 | 4.14 | 17.25 |
| 1 + b convex | −26.56 | −9.80 | 16.77 |
| 2 + b convex | −10.48 | 6.51 | 16.99 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mojica, M.; Méndez, F.; Alonso, J.A. Growth of Fullerene Fragments Using the Diels-Alder Cycloaddition Reaction: First Step towards a C60 Synthesis by Dimerization. Molecules 2013, 18, 2243-2254. https://doi.org/10.3390/molecules18022243
Mojica M, Méndez F, Alonso JA. Growth of Fullerene Fragments Using the Diels-Alder Cycloaddition Reaction: First Step towards a C60 Synthesis by Dimerization. Molecules. 2013; 18(2):2243-2254. https://doi.org/10.3390/molecules18022243
Chicago/Turabian StyleMojica, Martha, Francisco Méndez, and Julio A. Alonso. 2013. "Growth of Fullerene Fragments Using the Diels-Alder Cycloaddition Reaction: First Step towards a C60 Synthesis by Dimerization" Molecules 18, no. 2: 2243-2254. https://doi.org/10.3390/molecules18022243