Synthesis of Structured Lipids by Lipase-Catalyzed Interesterification of Triacetin with Camellia Oil Methyl Esters and Preliminary Evaluation of their Plasma Lipid-Lowering Effect in Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Comparison of Lipase
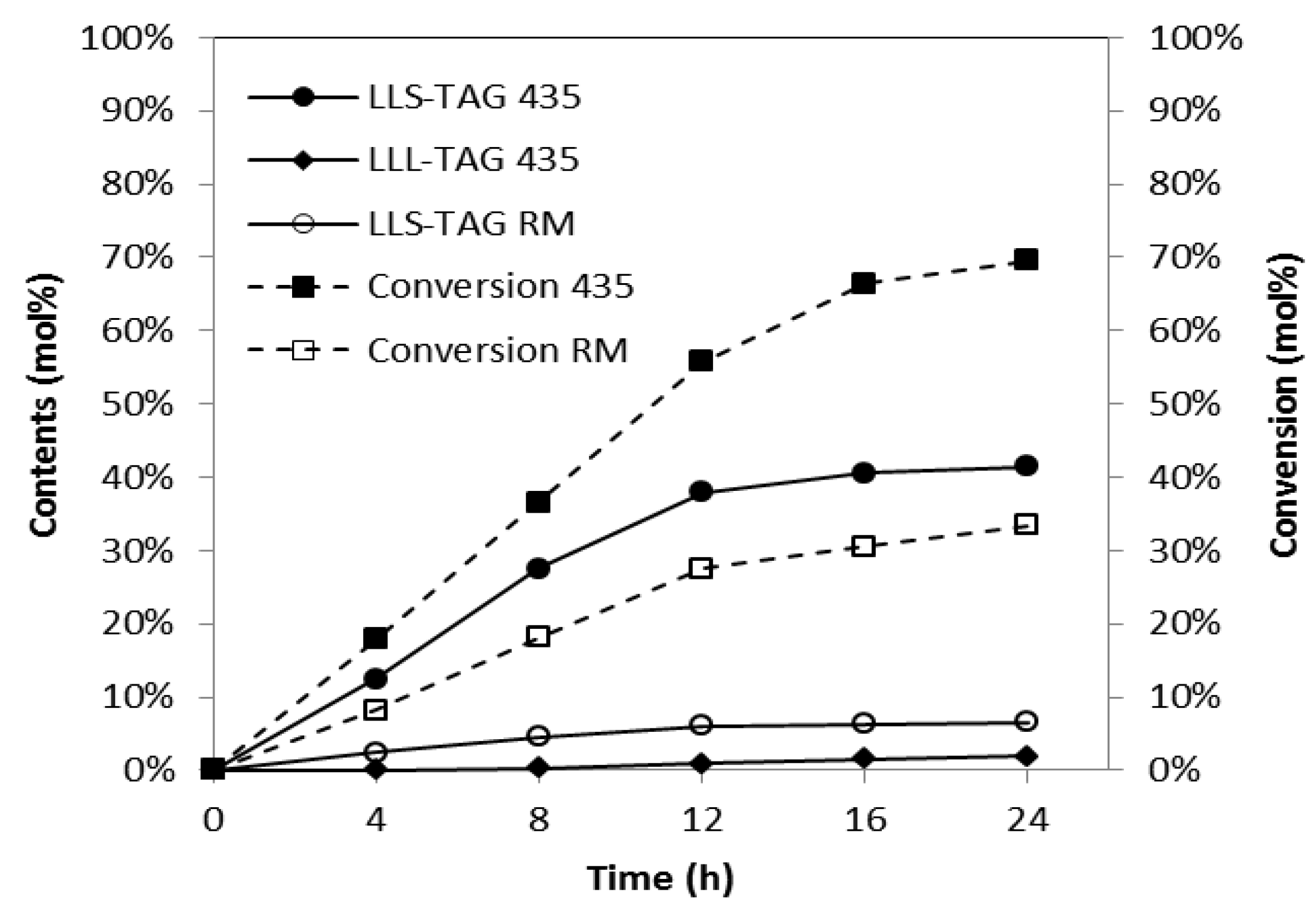
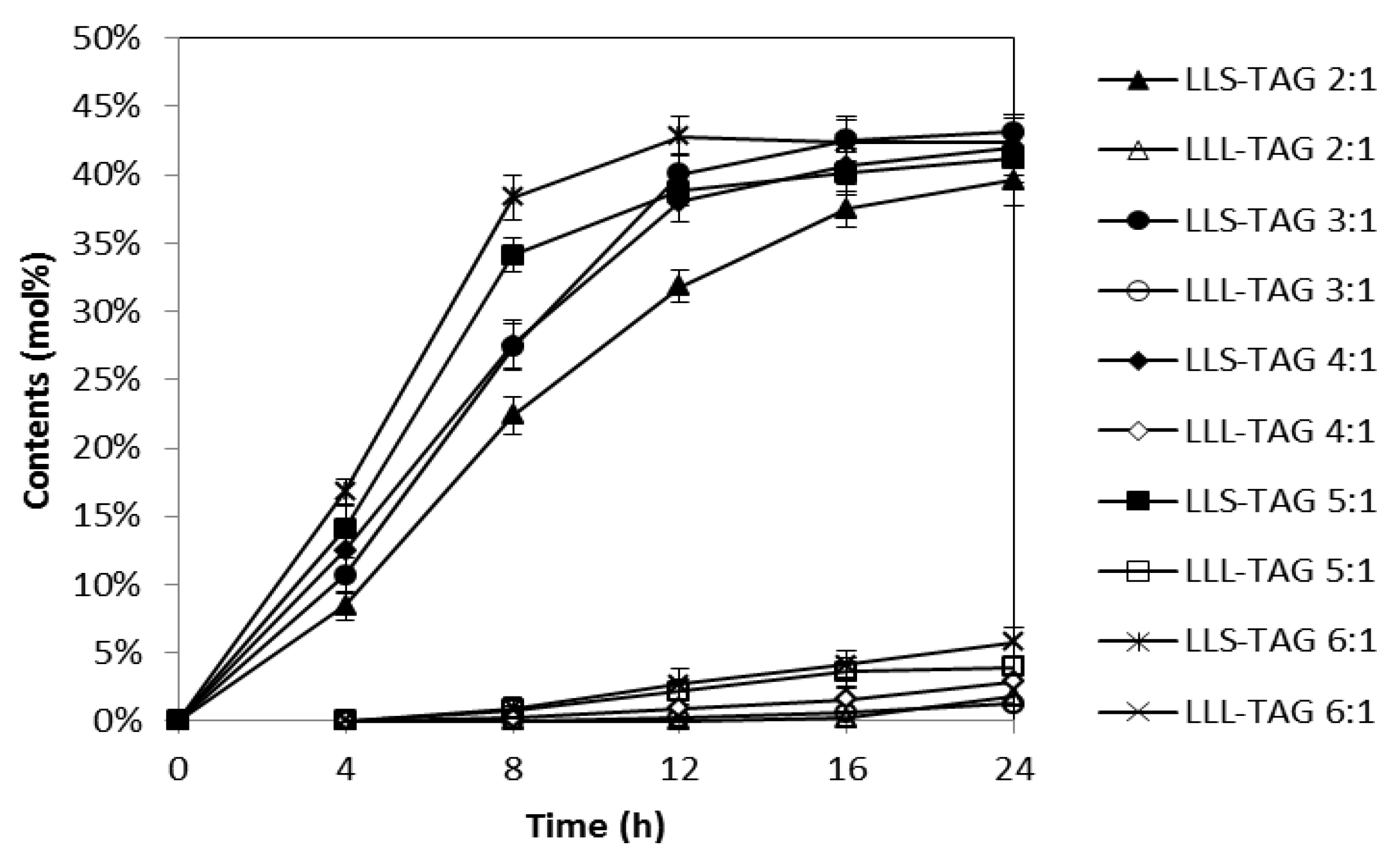
2.2. Effects of Substrate Molar Ratio
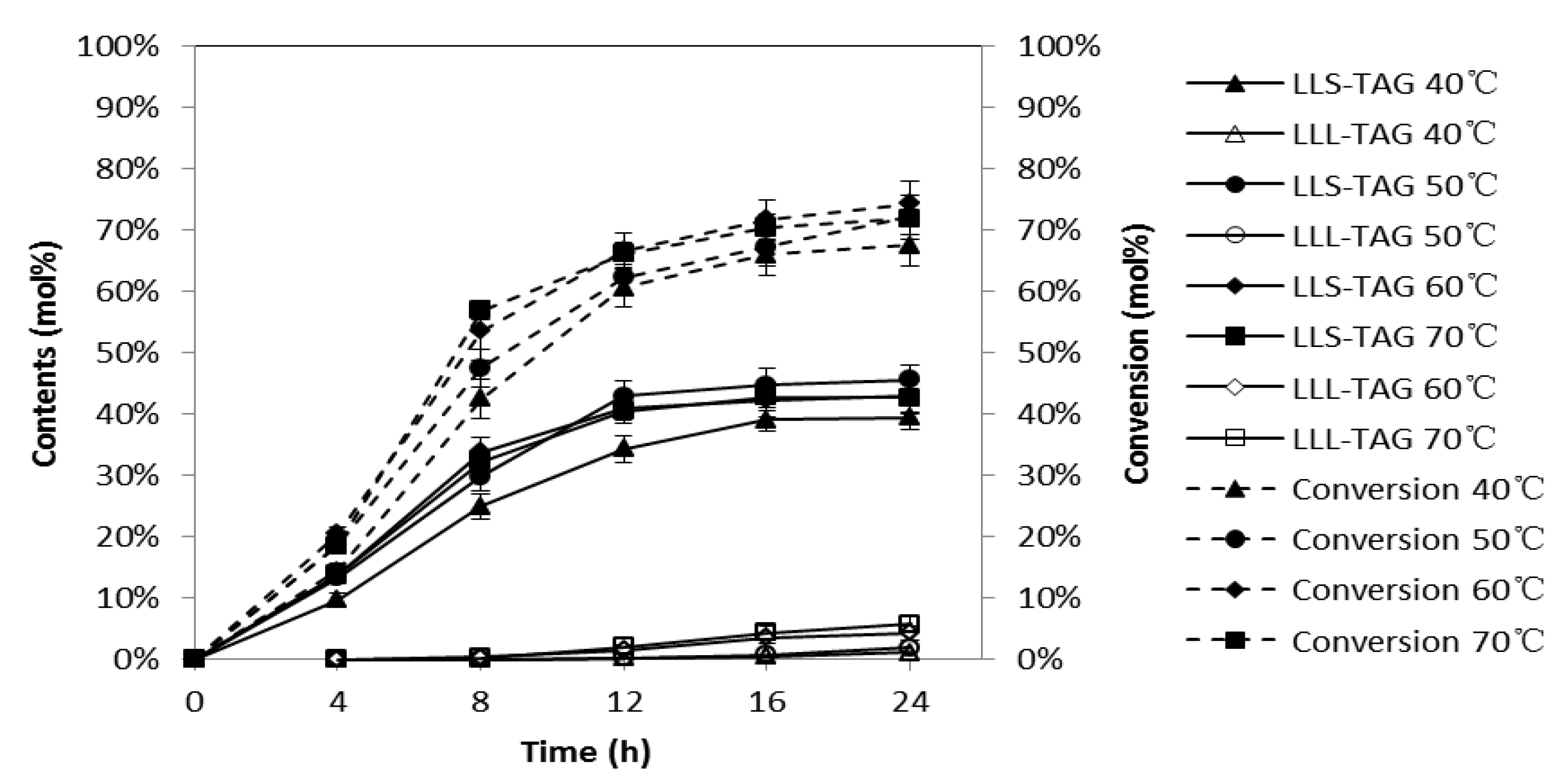
2.3. Effects of Reaction Temperature
2.4. Effects of Enzyme Loading

2.5. Scale-Up Synthesis and Purification of SLCTs
| Products | LLS-TAGs | LLL-TAGs | Others * |
|---|---|---|---|
| Interesterification product | 52.4 | 1.2 | 46.4 |
| MD distillates | 4.5 | 0.4 | 95.1 |
| MD residues | 94.6 | 4.4 | 1.0 |
2.6. Fatty Acid Composition
| Fatty acid | Fatty acid composition (mol%) | ||
|---|---|---|---|
| Camellia oil | DAG | SLCTs | |
| C2:0 | - * | - * | 29.16 ± 0.35 |
| C16:0 | 7.04 ± 0.65 | 7.05 ± 0.35 | 5.34 ± 0.43 |
| C18:0 | 2.72 ± 0.13 | 2.41 ± 0.19 | 2.00 ± 0.21 |
| C18:1 | 80.87 ± 1.74 | 81.21 ± 1.34 | 57.29 ± 0.49 |
| C18:2 | 8.48 ± 0.82 | 8.45 ± 1.08 | 5.59 ± 0.63 |
| C18:3 | 0.46 ± 0.20 | 0.51 ± 0.16 | 0.34 ± 0.04 |
| Others | 0.43 ± 0.06 | 0.37 ± 0.13 | 0.27 ± 0.05 |
| FFA (mg/g) | 0.48 ± 0.05 | - * | - * |
2.7. Plasma Analysis of Fasting Experiment
| Groups | TAG (mmol/L) | SLCTs (mmol/L) | DAG (mmol/L) | Blank (mmol/L) |
|---|---|---|---|---|
| TC | 4.05 ± 0.49 | 3.92 ± 0.99 | 3.17 ± 0.61 | 3.34 ± 0.60 |
| TG | 2.64 ± 0.43 a | 2.04 ± 0.40 a,b | 2.06 ± 0.28 a,b | 1.05 ± 0.10 |
| HDL-C | 1.54 ± 0.39 | 1.49 ± 0.47 | 1.25 ± 0.29 | 1.39 ± 0.21 |
| LDL-C | 10.26 ± 3.13 | 8.39 ± 3.51 | 8.11 ± 2.59 | 9.15 ± 2.55 |
3. Experimental
3.1. Materials
3.2. Comparison of Lipase
3.3. Synthesis of SLCTs
3.4. Analysis of Product
3.5. Fatty Acid Composition of SLs Product
3.6. Other Analyses
3.7. Scale-Up Synthesis and Purification of Product
3.8. Animals and Gastric Lavage Experiments
3.9. Serum Analysis
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Akoh, C.C. Structured Lipids-Enzymatic Approach. Inform 1995, 6, 1055–1061. [Google Scholar]
- Osborn, H.T.; Akoh, C.C. Structured Lipids—Novel fats with medical, nutraceutical, and food applications. Compr. Rev. Food Sci. Food Saf. 2002, 3, 110–120. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Tang, T.K.; Lai, O.M. Health Benefits, Enzymatic Production, and Application of medium- and long-chain triacylglycerol (MLCT) in food industries: A review. J. Food Sci. 2012, 77, R137–R144. [Google Scholar] [CrossRef]
- Foglia, T.A.; Villeneuve, P. Carica papaya latex-catalyzed synthesis of structured triacylglycerols. J. Am. Oil. Chem. Soc. 1997, 74, 1447–1450. [Google Scholar]
- Mangos, T.J.; Jones, K.C.; Foglia, T.A. Lipase-catalyzed synthesis of structured low-calorie triacylglycerols. J. Am. Oil. Chem. Soc. 1999, 76, 1127–1132. [Google Scholar]
- Yang, T.H.; Jang, Y.; Han, J.J.; Rhee, J.S. Enzymatic synthesis of low-calorie structured lipids in a solvent-free system. J. Am. Oil. Chem. Soc. 2000, 78, 291–296. [Google Scholar]
- Han, J.J.; Yamamoto, T. Enhancement of both reaction yield and rate of synthesis of structured triacylglycerol containing eicosapentaentaenoic acid under vacuum with water activity control. Lipids 1999, 34, 989–955. [Google Scholar] [CrossRef]
- Han, L.; Xu, Z.J.; Huang, J.H.; Meng, Z.; Liu, Y.F.; Wang, X.G. Enzymatically catalyzed synthesis of low-calorie structured lipid in a solvent-free system: optimization by response surface methodology. J. Agric. Food Chem. 2011, 59, 12635–12642. [Google Scholar]
- Lo, S.K.; Tan, C.P.; Yusoff, K.L.; Mohd, S.A.; Lai, O.M. Diacylglycerol oil-properties, processes and products: A review. Food Bioprocess Tech. 2008, 1, 223–233. [Google Scholar]
- Destaillats, F.; Craft, B.D.; Dubois, M.; Nagy, K. Glycidyl esters in refined palm (Elaeis guineensis) oil and related fractions. Part I: Formation mechanism. Food Chem. 2012, 2012, 1391–1398. [Google Scholar]
- Bakhiya, N.; Abraham, K.; Gürtler, R.; Appel, K.; Lampen, A. Toxicological assessment of 3-chloropropane-1,2-diol and glycidol fatty acid esters in food. Mol Nutr. Food Res. 2011, 4, 509–521. [Google Scholar]
- Zhang, D.L.; Stack, L.; Zhang, R.Q.; Yu, J.F.; Xie, B.X.; Chen, Y.Z.; Ruter, J.M. Tea oil camellia Eastern “Olive” for the world. Acta Hort. 2008, 769, 43–48. [Google Scholar]
- Grundy, S.M. Comparison of monounsaturated fatty acids and carbohydrates for lowering plasma cholesterol. New Engl. J. Med. 1986, 314, 745–748. [Google Scholar] [CrossRef]
- Gao, J. The importance of camellias as oil plants in China. Int. Camellia J. 1993, 25, 53–54. [Google Scholar]
- Karlstad, M.D.; Killeffer, J.A.; Bailey, J.W.; DeMichele, S.J. Parenteral nutrition with short- and long-chain triglycerides: triacetin reduces atrophy of small and large bowel mucosa and improves protein metabolism in burned rats. Am. J. Clin. Nutr. 1992, 55, 1005–1011. [Google Scholar]
- Bailey, J.W.; Barker, R.L.; Karlstad, M.D. Total parenteral nutrition with short- and long-chain triglycerides: triacetin improves nitrogen balance in rats. J. Nutr. 1992, 122, 1823–1829. [Google Scholar]
- Bloomer, S.; Adlercreutz, P.; Mattiasson, B. Triglyceride interesterification by lipases. 2. Reaction parameters for the reduction of trisaturate impurities and diglycerides in batch reactions. J. Biocatal. 1991, 5, 145–162. [Google Scholar]
- Tsuzuki, W. Acidolysis between triolein and short-chain fatty acid by lipase in organic solvents. Biosci. Biotechnol. Biochem. 2005, 69, 1256–1261. [Google Scholar] [CrossRef]
- Xua, X.; Balchen, S.; Høy, C.E.; Adler-Nissen, J. Pilot batch production of specific-structured lipids by lipase-catalyzed interesterification: preliminary study on incorporation and acyl migration. J. Am. Oil. Chem. Soc. 1998, 75, 301–308. [Google Scholar] [CrossRef]
- Xu, X.; Fomuso, L.; Akoh, C. Synthesis of structured triacylglycerols by lipase-catalyzed acidolysis in a packed bed bioreactor. J. Agric. Food Chem. 2000, 48, 3–10. [Google Scholar] [CrossRef]
- Hayes, J.R.; Finley, J.W.; Leveile, G.A. In vitro metabolism of SALATRIM fats in the rat. J. Agric. Food Chem. 1994, 42, 500–514. [Google Scholar] [CrossRef]
- Murase, T.; Aoki, M.; Wakisaka, T.; Tadashi Hase, T.; Tokimitsu, I. Anti-obesity effect of dietary diacylglycerol in C57BL/6J mice: dietary diacylglycerol stimulates intestinal lipid metabolism. J. Lipid. Res. 2002, 43, 1312–1319. [Google Scholar]
- Porsgaard, T.; Xu, X.; Göttsche, J.; Huiling, M. Differences in the intramolecular structure of structured oils do not affect pancreatic lipase activity in vitro or the absorption by rats of (n-3) fatty acids. J. Nutr. 2005, 135, 1705–1711. [Google Scholar]
- Stirton, A.J. Bailey’s industrial oil and fat products, 3rd ed.; Alive Books: New York, NY, USA, 1964. [Google Scholar]
- Wang, W.F.; Li, T.; Ning, Z.X.; Wang, Y.H.; Yang, B.; Yang, X.Q. Production of extremely pure diacylglycerol from soybean oil by lipase-catalyzed glycerolysis. Enzyme Microb. Tech. 2011, 49, 192–196. [Google Scholar] [CrossRef]
- Qin, X.L.; Wang, Y.M.; Wang, Y.H. Preparation and Characterization of 1, 3-Dioleoyl-2-palmitoylglycerol. J. Agric. Food Chem. 2011, 59, 5714–5719. [Google Scholar] [CrossRef]
- European Committee for Standardization. Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acid; ISO Method 12966-2; European Committee for Standardization: Brussels, Belgium, 2011. [Google Scholar]
- Wang, Y.H.; Mai, Q.Y.; Qin, X.L.; Yang, B.; Wang, Z.L.; Chen, H.T. Establishment of an evaluation model for human milk fat substitutes. J. Agric. Food Chem. 2010, 58, 642–649. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 4th ed.; AOCS Press: Champaign, IL, USA, 1993; Methods Ca–5a. [Google Scholar]
- Sample Availability: Sample of the compound LLS-TAG is available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cao, Y.; Qi, S.; Zhang, Y.; Wang, X.; Yang, B.; Wang, Y. Synthesis of Structured Lipids by Lipase-Catalyzed Interesterification of Triacetin with Camellia Oil Methyl Esters and Preliminary Evaluation of their Plasma Lipid-Lowering Effect in Mice. Molecules 2013, 18, 3733-3744. https://doi.org/10.3390/molecules18043733
Cao Y, Qi S, Zhang Y, Wang X, Yang B, Wang Y. Synthesis of Structured Lipids by Lipase-Catalyzed Interesterification of Triacetin with Camellia Oil Methyl Esters and Preliminary Evaluation of their Plasma Lipid-Lowering Effect in Mice. Molecules. 2013; 18(4):3733-3744. https://doi.org/10.3390/molecules18043733
Chicago/Turabian StyleCao, Yu, Suijian Qi, Yang Zhang, Xiaoning Wang, Bo Yang, and Yonghua Wang. 2013. "Synthesis of Structured Lipids by Lipase-Catalyzed Interesterification of Triacetin with Camellia Oil Methyl Esters and Preliminary Evaluation of their Plasma Lipid-Lowering Effect in Mice" Molecules 18, no. 4: 3733-3744. https://doi.org/10.3390/molecules18043733




