Comparative Study on the Larvicidal Activity of Drimane Sesquiterpenes and Nordrimane Compounds against Drosophila melanogaster til-til
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
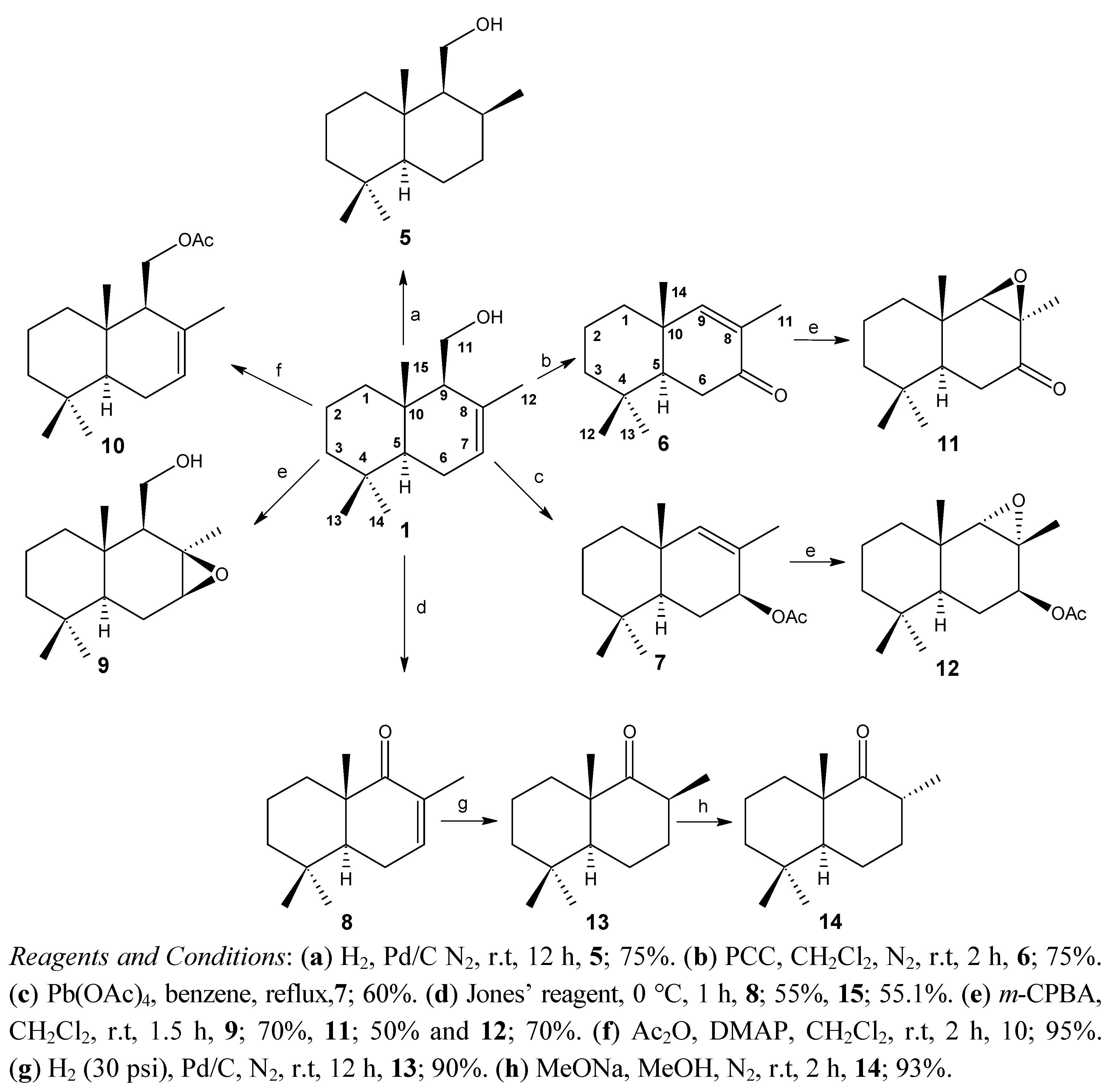
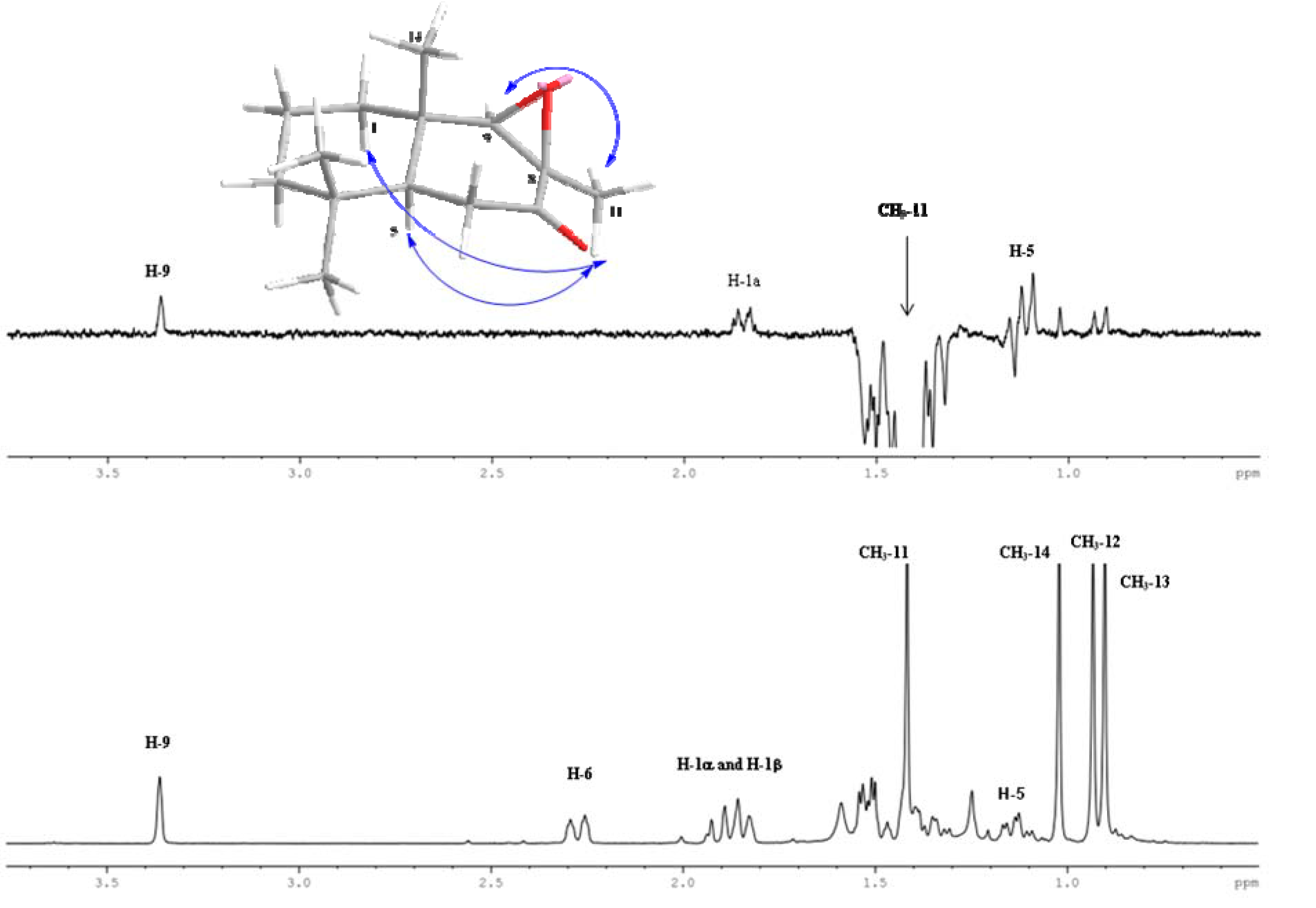

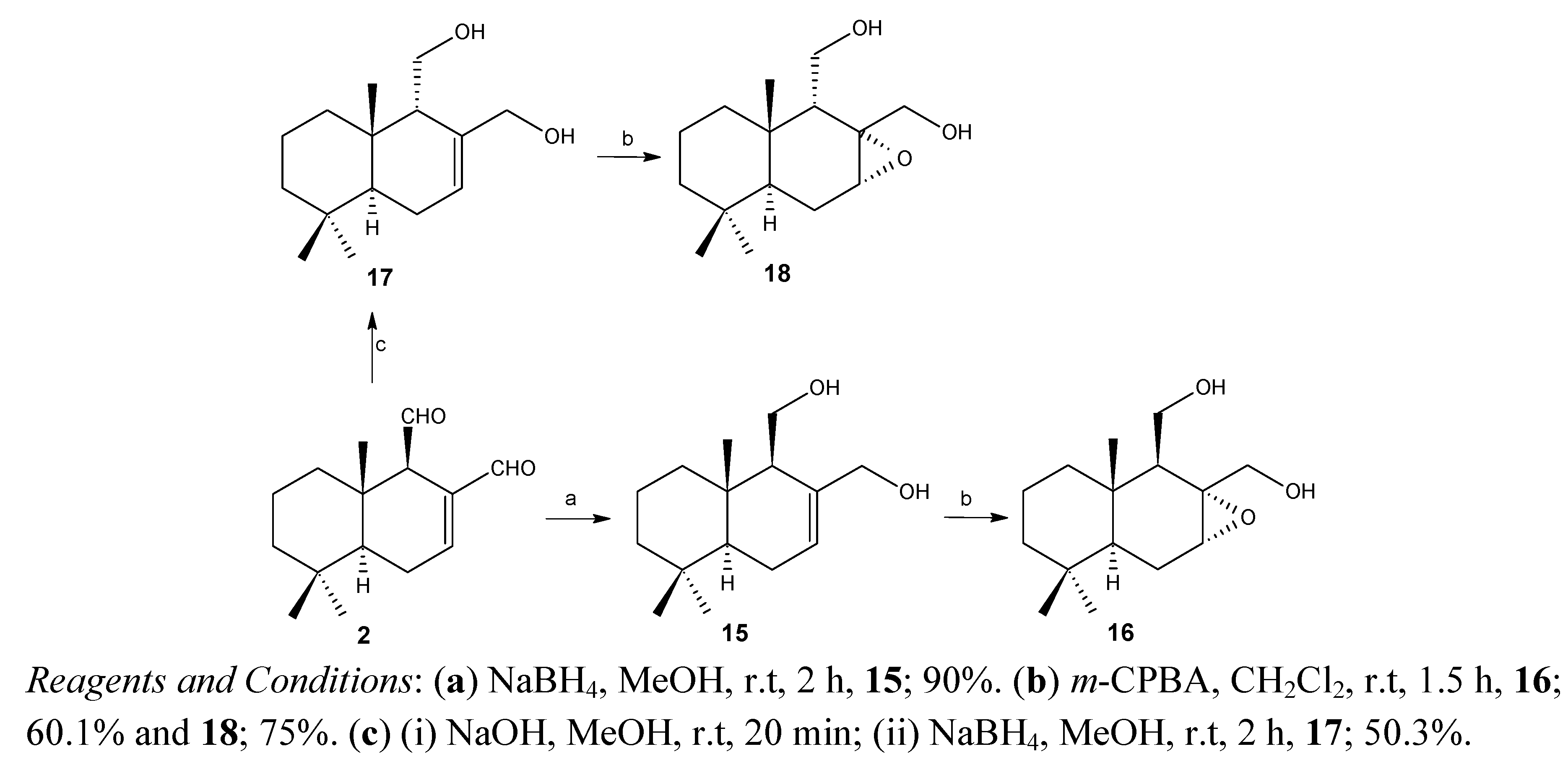
2.2. Biological Results
2.3. Antifeedant Activity No-Choice Test
| Concentration (ppm) | AI ** (Mean ± SD) | 1 | 3 | 4 | 15 | 16 | 17 |
|---|---|---|---|---|---|---|---|
| Control solvent | 0.0 | ||||||
| 5 | 51.82 ± 6.7 | 52.25 ± 5.4 | 53.00 ± 0.9 | 44.79 ± 3.3 | 53.98 ± 3.6 | 77.51 ± 2.7 | |
| 25 | 45.18 ± 3.4 | 43.77 ± 6.2 | 31.55 ± 4.2 | 37.68 ± 3.6 | 29.46 ± 3.9 | 59.41 ± 1.7 | |
| 50 | 34.27 ± 3.0 | 32.63 ± 2.3 | 32.63 ± 5.3 | 24.98 ± 6.3 | 41.63 ± 4.2 | ||
| 100 | 18.17 ± 1.2 | 16.40 ± 1.9 | 16.50 ±2.8 | 20.80 ± 2.2 | 31.32 ± 2.8 | ||
| * Safrole 5 | 33.21 ± 0.8 | ||||||
| * Azadirachtin 5 | 53.26 ± 1.6 |
2.4. Larvicidal Activity against D. melanogaster
| Compounds | % Mortality | EC50 (mg/L) |
|---|---|---|
| 1 | 15.0 ± 0.3 | >100 |
| 2 | 50.0 ± 0.4 | 60.0 ± 4.2 |
| 3 | 4.10 ± 0.9 | >100 |
| 4 | 100 ± 0.2 | 4.5 ± 0.8 |
| 5 | 0.0 | >100 |
| 6 | 4.2 ± 1.2 | >100 |
| 7 | 33.3 ± 1.1 | 57.8 ± 2.3 |
| 8 | 0.0 | >100 |
| 9 | 0.0 | >100 |
| 10 | 0.0 | >100 |
| 11 | 0.0 | >100 |
| 12 | 12.5 ± 1.2 | 83.2 ± 3.5 |
| 13 | 0.0 | >100 |
| 14 | 0.0 | >100 |
| 15 | 46.2 ± 1.3 | 45.0 ± 2.8 |
| 16 | 35.3 ± 0.2 | 70.0 ± 2.6 |
| 17 | 35.0 ± 1.1 | 70.9 ± 3.7 |
| 18Safrole *Azadirachtin * | 0.020.0 ± 1.636.7 ± 0.5 | >10018.0 ± 1.515.0 ± 1.1 |
2.5. Growth Inhibition and Relative Growth Index for D. melanogaster til-til
| Compounds | Concentration(ppm) | GIx RGIz | |
|---|---|---|---|
| Control solventSafrole *1Azadirachtin *2 | 0.05.05.0 | 0.95 ± 0.03 x 0.40 ± 0.02 x 0.19 ± 0.03 x | 1.00.470.20 |
| 2 | 25.050.0100.0250.0500.0 | 0.92 ± 0.04 y0.86 ± 0.05 y0.79 ± 0.08 y0.61 ± 0.19 y0.32 ± 0.09 y | 0.970.910.830.640.34 |
| 4 | 5.025.050.0 * | 0.25 ± 0.03 y0.11 ± 0.09 y- | 0.260.12- |
| 7 | 25.050.0100.0250.0500.0 | 0.95 ± 0.12 y0.90 ± 0.13 y0.73 ± 0.10 y0.62 ± 0.09 y0.29 ± 0.06 y | 1.00.950.770.650.31 |
| 15 | 25.050.0100.0250.0500.0 | 0.63 ± 0.07 y0.50 ± 0.09 y0.35 ± 0.12 y0.15 ± 0.04 y0.09 ± 0.05 y | 0.660.530.370.160.09 |
| 17 | 25.050.0100.0 | 0.56 ± 0.12 y0.48 ± 0.10 y0.31 ± 0.06 y- | 0.590.500.33- |
| Treatment | Doses(mg/L) | Mean weight gained (mg) c | % d | Pupation(SP) e | Emergence(%) f | Mortality(%) |
|---|---|---|---|---|---|---|
| Control solvent | 0.0 | 0.813 ± 0.15 a | 100 | 100 | 100 | 0 |
| Safrole *Azadirachtin *1 | 55550100 | 0.270 ± 0.16 a0.433 ± 0.13 a0.258 ± 0.14 a0.398 ± 0.17 a0.563 ± 0.18 a | 33.253.332.049.069.0 | 10047.570.078.380.6 | 10063.378.076.377.4 | 20.036.715.0 |
| 2 | 550 | 0.59 ± 0.16 a0.647 ± 0.14 a | 72.679.6 | 72.780.0 | 98.097.0 | 50.0 |
| 100 | 0.698 ±0.12 a | 85.9 | 86.0 | 98.0 | ||
| 5 | 0.255 ± 0.13 a | 31.4 | 80.0 | 70.0 | ||
| 3 | 50 | 0.413 ± 0.14 a | 50.8 | 85.0 | 85.0 | 4.1 |
| 100 | 0.587 ± 0.11 a | 72.2 | 80.0 | 80.0 | ||
| 5 | 0.250 ± 0.10 a | 30.7 | 45.0 | 46.7 | ||
| 4 | 50 | 0 | 0 | 0 | 0 | 100 |
| 100 | 0 | 0 | 0 | 0 | ||
| 5 | 0.810 ± 0.10 a | 99.6 | 80.0 | 78.3 | ||
| 6 | 50 | 0.797 ± 0.13 a | 98.0 | 80.0 | 93.5 | 4.2 |
| 100 | 0.800 ± 0.12 a | 98.4 | 85.0 | 86.7 | ||
| 5 | 0.801 ± 0.08 a | 98.5 | 91.6 | 95.0 | ||
| 7 | 50 | 0.763 ± 0.09 a | 93.8 | 95.0 | 95.0 | 33.3 |
| 100 | 0.750 ± 0.09 a | 92.3 | 73.3 | 97.8 | ||
| 5 | 0.803 ± 0.09 a | 98.8 | 100 | 98.3 | ||
| 12 | 50 | 0.793 ± 0.10 b | 97.5 | 100 | 91.7 | 12.5 |
| 100 | 0.769 ± 0.12 a | 94.6 | 100 | 91.7 | ||
| 5 | 0.310± 0.08 a | 38.3 | 80.0 | 80.0 | ||
| 15 | 50 | 0.413± 0.08 a | 50.8 | 70.0 | 80.0 | 46.2 |
| 100 | 0.587 ± 0.06 a | 72.2 | 95 | 80.0 | ||
| 5 | 0.243 ± 0.12 a | 29.8 | 60.0 | 60.0 | ||
| 16 | 50 | 0.443 ± 0.08 b | 54.5 | 91.6 | 90.0 | 35.3 |
| 100 | 0.533 ± 0.08 a | 65.5 | 58.3 | 50.5 | ||
| 5 | 0.103 ± 0.11 a | 12.6 | 93.3 | 93.5 | ||
| 17 | 50 | 0.207 ± 0.12 a | 25.4 | 85.0 | 85.0 | 35.0 |
| 100 | 0.480 ± 0.15 a | 59.0 | 65.0 | 91.5 |
3. Experimental
3.1. General
3.2. Plant Material
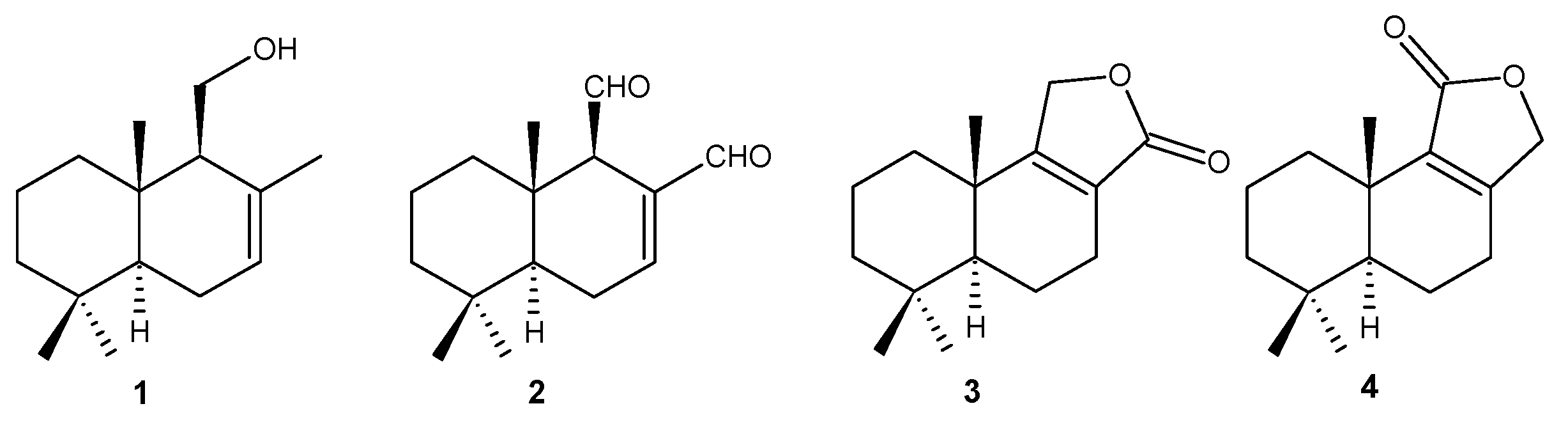
3.3. Obtainment of Natural Compounds 1–4
3.4. Preparation of Drimenol Derivatives 5–14
3.5. Preparation of Polygodial Derivatives 15–18
3.6. Fly Strains
3.7. No-Choice Test (Insecticidal Bioassay) against D. melanogaster
3.8. Relative Growth Index and Growth Index
3.8.1. Antifeedant Test against D. melanogaster
3.8.2. Statistical Analyses
4. Conclusions
Acknowledgments
References
- Flores, S.V.; Iturra, P.; Godoy-Herrera, R. Genetic differentiation between three populations of Drosophila pavani that live in different breeding sites in nature. Dros. Inf. Serv. 2001, 84, 67–70. [Google Scholar]
- Flores, S.; Flores, L.; Godoy-Herrera, R. Molecular phylogeny of the mesophragmatica species group inferred from cytochrome Oxidase II sequence. Dros. Inf. Serv. 2003, 85, 76–78. [Google Scholar]
- Flores, S. Biología Evolutiva de especies Chilenas de Drosophila con descripción de una nueva especie del género. Ph.D. Thesis, Joint Doctoral Program, Universidad de Chile, Santiago, Chile, 2009. [Google Scholar]
- Appel, H.H. Estudio químico de la corteza del árbol Drimys. Winteri.Forst. Scientia. 1948, 15, 31–32. [Google Scholar]
- Jansen, B.J.M.; de Groot, A. The occurrence and biological activity of drimane sesquiterpenoids. Nat. Prod. Rep. 1991, 8, 309–318. [Google Scholar] [CrossRef]
- Matsuda, H.; Pongpiriyadacha, Y.; Morikawa, T.; Kashima, Y.; Nakano, K.; Yoshikawa, M. Protective effects of polygodial and related compounds on ethanol-induced gastric mucosal lesions in rats: Structural requirements and mode of action. Bioorg. Med. Chem. Lett. 2002, 12, 477–482. [Google Scholar] [CrossRef]
- Anke, H.; Sterner, O. Comparison of the antimicrobial and cytotoxic activities of twenty unsaturated sesquiterpene dialdehydes from plants and mushrooms. Planta. Med. 1991, 57, 344–346. [Google Scholar] [CrossRef]
- Akasawa, Y.; Dawson, G.W.; Griffihs, D.C.; Lallemand, J.Y.; Ley, S.V.; Mori, K.; Mudd, A.; Pezechk-Leclaire, M.; Pickett, J.A.; Watanabe, H.; et al. Activity of drimane antifeedants and related compounds against aphids, and comparative biological effects and chemical reactivity of (−) and (+)-polygodial. J. Chem. Ecol. 1988, 14, 1845–1855. [Google Scholar] [CrossRef]
- Marston, A.; Hostettmann, K. Plant molluscicides. Phytochemistry 1985, 24, 639–652. [Google Scholar] [CrossRef]
- Cortes, M.; Razmilic, I.; López, J. Synthesis of (−) Ugandensidial. J. Nat. Prod. 1990, 53, 1369–1371. [Google Scholar] [CrossRef]
- Jansen, B.J.M; de Groot, A. Occurrence, Biological activity and synthesis of drimane sesquiterpenoids. Nat. Prod. Rep. 2004, 21, 449–477. [Google Scholar] [CrossRef]
- Cooney, L.J.; van Klink, J.W.; Hughes, N.M.; Perry, N.B.; Schaefer, H.M.; Menzies, I.J.; Gould, K.S. Red leaf margins indicate increased polygodial content and function as visual signals to reduce herbivory in Pseudowintera colorata. New Phytologist. 2012, 194, 488–497. [Google Scholar] [CrossRef]
- Gerard, P.J.; Perry, N.B.; Ruf, L.D.; Foster, L.M. Antifeedant and insecticidal activity of compounds from Pseudowintera colorata (Winteraceae) on the webbing clothes moth, Tineola bisselliella (Lepidoptera: Tineidae) and the Australian carpet beetle, Anthrenocerus australis (Coleoptera: Dermestidae). Bull. Entomol. Res. 1993, 83, 547–552. [Google Scholar] [CrossRef]
- Sipos, E.; Fogassy, G.; Samant, P.V.; Figueiredo, J.L.; Tungler, A. Catalysis of Organic Reactions, Chapter 59. In Enantioselective Hydrogenations of Exo- and Endocyclic C=C Double Bond with Highly Mesoporous Carbon Supported Pd.; John, R., Sowa, Jr., Eds.; CRC Press: Porto, Portugal, 2005; pp. 525–534. [Google Scholar]
- Gesson, J.P.; Jacquesy, J.C.; Renoux, B. A new annulation of carvone to chiral trans and cis fused bicyclic ketones. Tetrahedron Lett. 1986, 37, 4461–4464. [Google Scholar] [CrossRef]
- Hlubucek, J.R.; Aasen, A.J.; Kimland, B.; Enzell, C.R. New volatile constituents of Greek Nicotiana tabacum. Phytochemistry 1973, 12, 2555–2557. [Google Scholar] [CrossRef]
- Brooks, C.J.W.; Lindsay, G.; Overton, K.H. A revised structure for nordrimenone. J. Chem. Soc.C 1970, 203–204. [Google Scholar]
- Hersel, U.; Steck, M.; Seifert, K. A New Route to 2,7- and 7-Functionalized Labdanes. Eur. J. Org. Chem. 2000, 8, 1609–1615. [Google Scholar] [CrossRef]
- Cheung, W.S.; Wong, H.N.C. A Total Synthesis of (+/−)-Hispanolone. Tetrahedron Lett. 1998, 39, 6521–6524. [Google Scholar]
- Dastlik, K.A.; Ghisalberti, E.L.; Skelton, B.W.; White, A.H. Structural Study of (−)-8-Epi-11-nordriman-9-one. Aust. J. Chem. 1991, 44, 123–127. [Google Scholar]
- Abad, A.; Agulló, C.; Castelblanque, L.; Cuñat, A.C.; Navarro, I.; Ramírez de Arellano, M.C. Synthesis of Terpenoid Unsaturated 1,4-Dialdehydes. π-Facial Selectivity in the Diels-Alder Reaction of the 1-Vinyl-2-methylcyclohexene Moiety of Polycyclic Systems with DMAD. J. Org. Chem. 2000, 65, 4189–4192. [Google Scholar] [CrossRef]
- Cuellar, M.A.; Moreno, L.E.; Preite, M.D. Regioselective oxidative fragmentation of drimanic terpene alcohols: a short, easy and efficient access to natural and synthetic 11-nordrimane terpene derivatives. ARKIVOC 2003, 10, 169–177. [Google Scholar] [CrossRef]
- Panasenko, A.; Gorincioi, E.C.; Aricu, A.N.; Barcari, E.A.; Deleanu, K.; Vlada, P.F. 1H and 13C-NMR spectra of some drimanic sesquiterpenoids. Russ. Chem. Bull. Int. Ed. 2004, 53, 2700–2705. [Google Scholar] [CrossRef]
- Moreno-Osório, L.; Cortés, M.; Armstrong, V.; Bailén, M.; González-Coloma, A. Antifeedant Activity of Some Polygodial Derivatives. Z. Naturforsch. C 2008, 63, 215. [Google Scholar]
- Cortés, M.; Moreno, L.;José, López. Partial Synthesis of (-)-11, 12-Dinordriman-8-one and the (−)-Enantiomer of Polywood. J. Chem. Res. Synop. 1998, 1, 36–37. [Google Scholar]
- Messchendorp, L.; Gols, G.J.Z.; van Loon, J.J.A. Behavioural observations of Pieris brassicae larvae indicate multiple mechanisms of action of analogous drimane antifeedants. Entomol. Exp.Appl. 2000, 95, 217–227. [Google Scholar]
- Messchendorp, L.; van Loon, J.J.A.; Gols, G.J.Z. Behavioural and sensory responses to drimane antifeedants in Pieris brassicae larvae. Entomol. Exp. Appl. 1996, 79, 195–202. [Google Scholar] [CrossRef]
- Fritz, G.L.; Mills, G.D.; Warhen, J.D.; Waters, R.M. Reimer-Tiemann adducts as potential insect antifeedant agents-Reviewing the structure activity relationship theory of the antifeedant, Warburganal. J. Chem. Ecol. 1989, 15, 2607–2623. [Google Scholar] [CrossRef]
- Jansen, B.J.M.; de Groot, A. The synthesis of drimane sesquiterpenoids. Nat. Prod. Rep. 1991, 8, 319–337. [Google Scholar] [CrossRef]
- Misra, J.R.; Horner, M.A.; Lam, G.; Thummel, C.S. Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes. Dev. 2011, 25, 1796–1806. [Google Scholar] [CrossRef]
- Daborn, P.J.; Lumb, C.; Boey, A.; Wong, W.; Ffrench-Constant, RH.; Batterham, P. Evaluating the insecticide resistance potential of eight Drosophila melanogaster cytochrome P450 genes by transgenic over-expression. Insect Biochem. Mol. Biol. 2007, 37, 512–519. [Google Scholar] [CrossRef]
- Daborn, P.J.; Yen, J.L.; Bogwitz, M.R.; Le Goff, G.; Feil, E.; Jeffers, S.; Tijet, N.; Perry, T.; Heckel, D.; Batterham, P.; et al. A single P450 allele associated with insecticide resistance in Drosophila. Science 2002, 297, 2253–2256. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar]
- Sykiotis, G.P.; Bohmann, D. Stress-activated cap’n’collar transcription factors in aging and human disease. Sci. Signal. 2010, 3, 112. [Google Scholar]
- Moreno-dorado, F.J.; Guerra, F.M.; Aladro, F.J.; Bustamante, J.M.; Massanet, G.M. Synthesis of (±)-11-hydroxy-3-oxo-6H, 7H, 10Me-eudesman-1,2-4,5-dien-6,12olide. J. Nat. Prod. 2000, 63, 934–938. [Google Scholar]
- Zhang, Q.; Pi, J.; Woods, C.G.; Andersen, M.E. Phase I to II Cross-Induction of Xenobiotic Metabolizing Enzymes: A Feedforward Control Mechanism for Potential Hormetic Responses. Toxicol. Appl. Pharmacol. 2009, 237, 345–356. [Google Scholar] [CrossRef]
- Perry, T.; Batterham, P.; Daborn, P.J. The biology of insecticidal activity and resistance. Insect Biochem. Biol. Mol. 2011, 41, 411–422. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. The frequency of U-shaped dose responses in the toxicological literature. Toxicol. Sci. 2001, 62, 330–338. [Google Scholar] [CrossRef]
- Deritaa, M.G.; Leivab, M.L.; Zacchino, S.A. Influence of plant part, season of collection and content of the main active constituent, on the antifungal properties of Polygonum acuminatum Kunt. J. Ethnopharmacol. 2009, 124, 377–383. [Google Scholar] [CrossRef]
- Derita, M.G.; Gattuso, S.J.; Zacchino, S. Occurrence of polygodial in species of Polygonum genus belonging to Persicaria section. Biochem. Syst. Ecol. 2008, 36, 55–58. [Google Scholar]
- Rodríguez, B.; Zapata, N.; Medina, P.; Viñuela, E. A complete 1H and 13C-NMR data assignment for four drimane sesquiterpenoids isolated from Drimys. winteri. Magn. Reson. Chem. 2005, 43, 82–84. [Google Scholar] [CrossRef]
- Hueso-Rodríguez, J.; Rodríguez, B. A new and efficient route to optically active drimanes. Synthesis of (+)-winterin, (+)-confertifolin, (+)-isodrimenin, and (+)-bicyclofarnesol. Tetrahedron 1989, 45, 1567–1576. [Google Scholar] [CrossRef]
- Cortés, M.; Delgado, V.; Saitz, C; Armstrong, V. Drimenol: A Versatile Synthon for Compounds with trans-Drimane Skeleton. Nat. Prod. Comm. 2011, 6, 477–490. [Google Scholar]
- Mihm, J.A. Mass rearing stem borers, fall armyworms and corn earworms at 675 CIMMYT. In Toward Insect Resistant Maize for the Third World, Proceedings of the International Symposium on Methodologies for Developing Host Plant 677 Resistance to Maize, CIMMYT-Mexico, Mexico D.F., Mexico, 1989; pp. 5–21.
- Muñoz, E.; Lamilla, C.; Marin, J.C.; Alarcon, J.; Cespedes, C.L. Antifeedant, insect growth regulatory and insecticidal effects of Calceolaria talcana (Calceolariaceae) on Drosophila melanogaster and Spodoptera frugiperda. Ind. Crop. Prod. 2013, 42, 137–144. [Google Scholar] [CrossRef]
- Zhang, M.; Chaudhuri, S.K.; Kubo, I. Quantification of insect growth and its use in screening of naturally ocurring insect control agents. J. Chem. Ecol. 1993, 19, 1109–1118. [Google Scholar] [CrossRef]
- Kiełczewski, M.; Drożdż, B.; Nawrot, J. Badania nad repelentami pokarmowymi trojszyka ulca (Tribolium confusum Duv.). Materiały XIX Sesji Naukowej Instytutu Ochrony Roślin; Państwowe Wydaw. Rolnicze i Leśne: Oddział, Poland, 1979; pp. 367–376. [Google Scholar]
- Lipa, J.J.; Wiland, E. Bacteria isolated from cutworms and their infectivity to Agrotis sp. Acta Microbiologica Polonica 1972, 4, 127–140. [Google Scholar]
- Thiery, I.; Frachon, E. Identification, isolation, culture and preservation of enthomopathogenic bacteria. In Manual of Techniques in Insect Pathology; Lawrence, A.L., Ed.; Academic Press: London, UK, 1999; pp. 55–73. [Google Scholar]
- Sample Availability: Samples of the compounds 1–18 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Montenegro, I.; Pino, L.; Werner, E.; Madrid, A.; Espinoza, L.; Moreno, L.; Villena, J.; Cuellar, M. Comparative Study on the Larvicidal Activity of Drimane Sesquiterpenes and Nordrimane Compounds against Drosophila melanogaster til-til. Molecules 2013, 18, 4192-4208. https://doi.org/10.3390/molecules18044192
Montenegro I, Pino L, Werner E, Madrid A, Espinoza L, Moreno L, Villena J, Cuellar M. Comparative Study on the Larvicidal Activity of Drimane Sesquiterpenes and Nordrimane Compounds against Drosophila melanogaster til-til. Molecules. 2013; 18(4):4192-4208. https://doi.org/10.3390/molecules18044192
Chicago/Turabian StyleMontenegro, Ivan, Luis Pino, Enrique Werner, Alejandro Madrid, Luis Espinoza, Luis Moreno, Joan Villena, and Mauricio Cuellar. 2013. "Comparative Study on the Larvicidal Activity of Drimane Sesquiterpenes and Nordrimane Compounds against Drosophila melanogaster til-til" Molecules 18, no. 4: 4192-4208. https://doi.org/10.3390/molecules18044192





