H/D Isotope Effects in Hydrogen Bonded Systems
1. Introduction
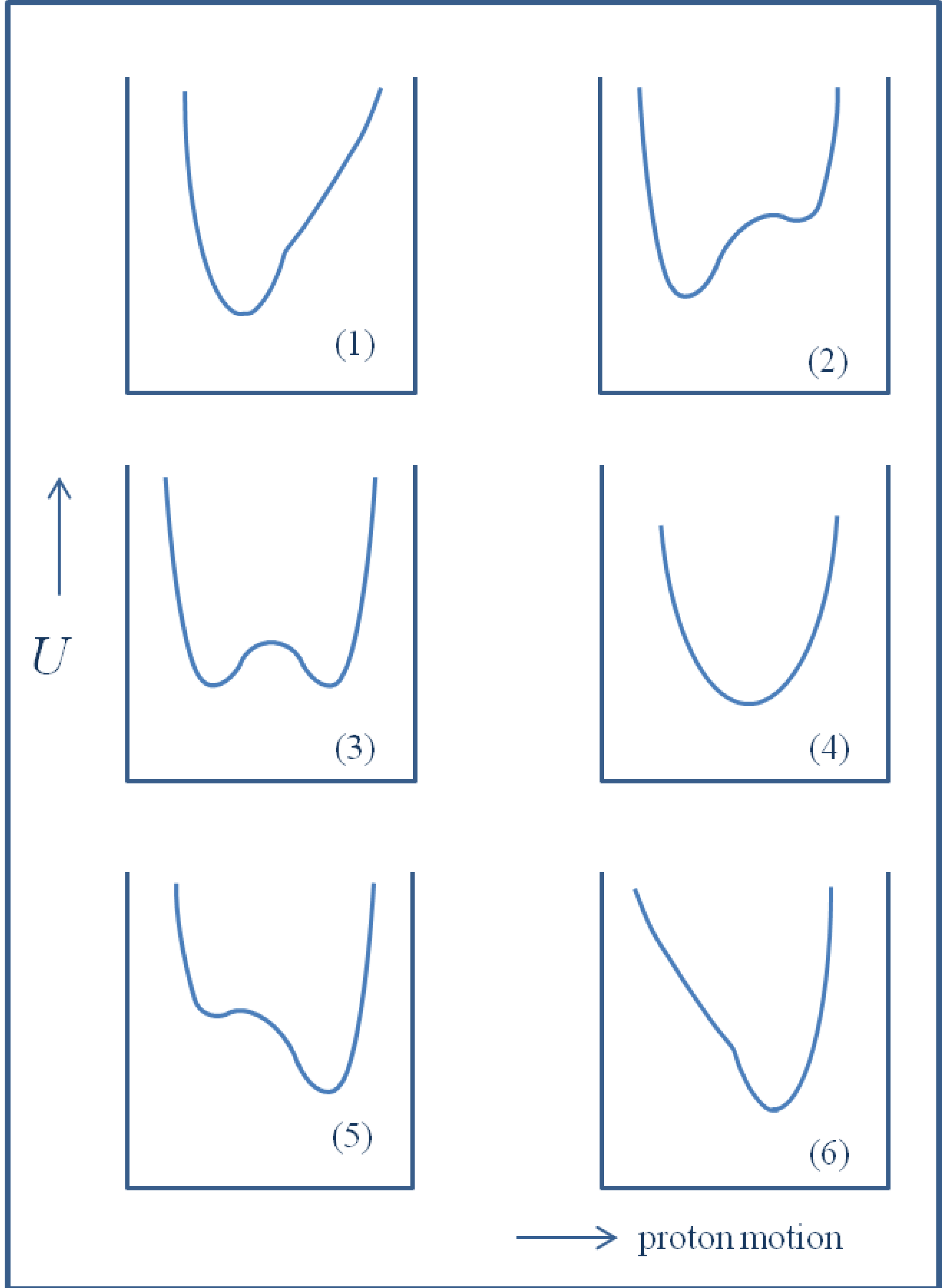
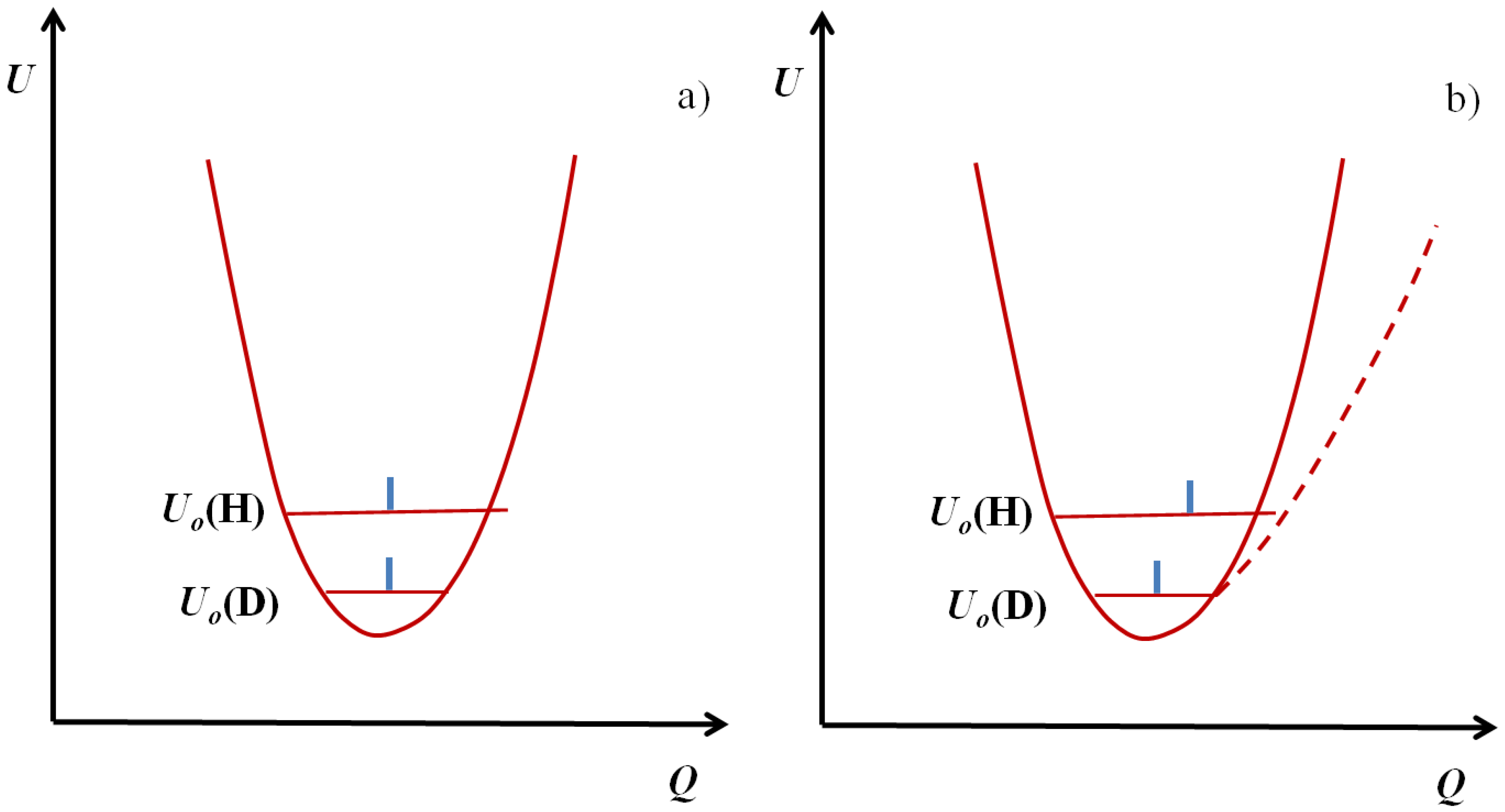
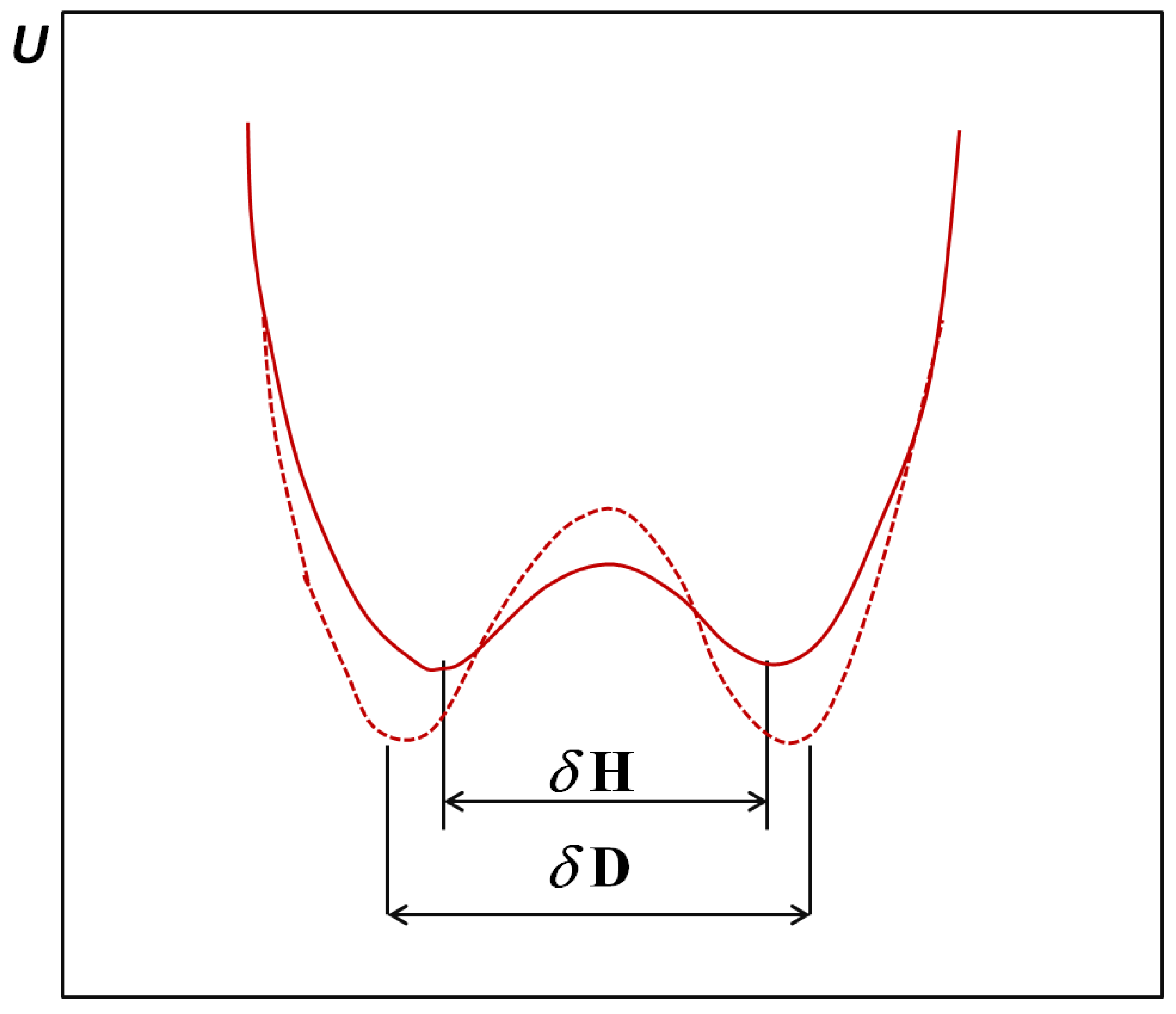
2. H/D Isotope Effects in Hydrogen Bonds Reflected in Infrared Absorption Spectra
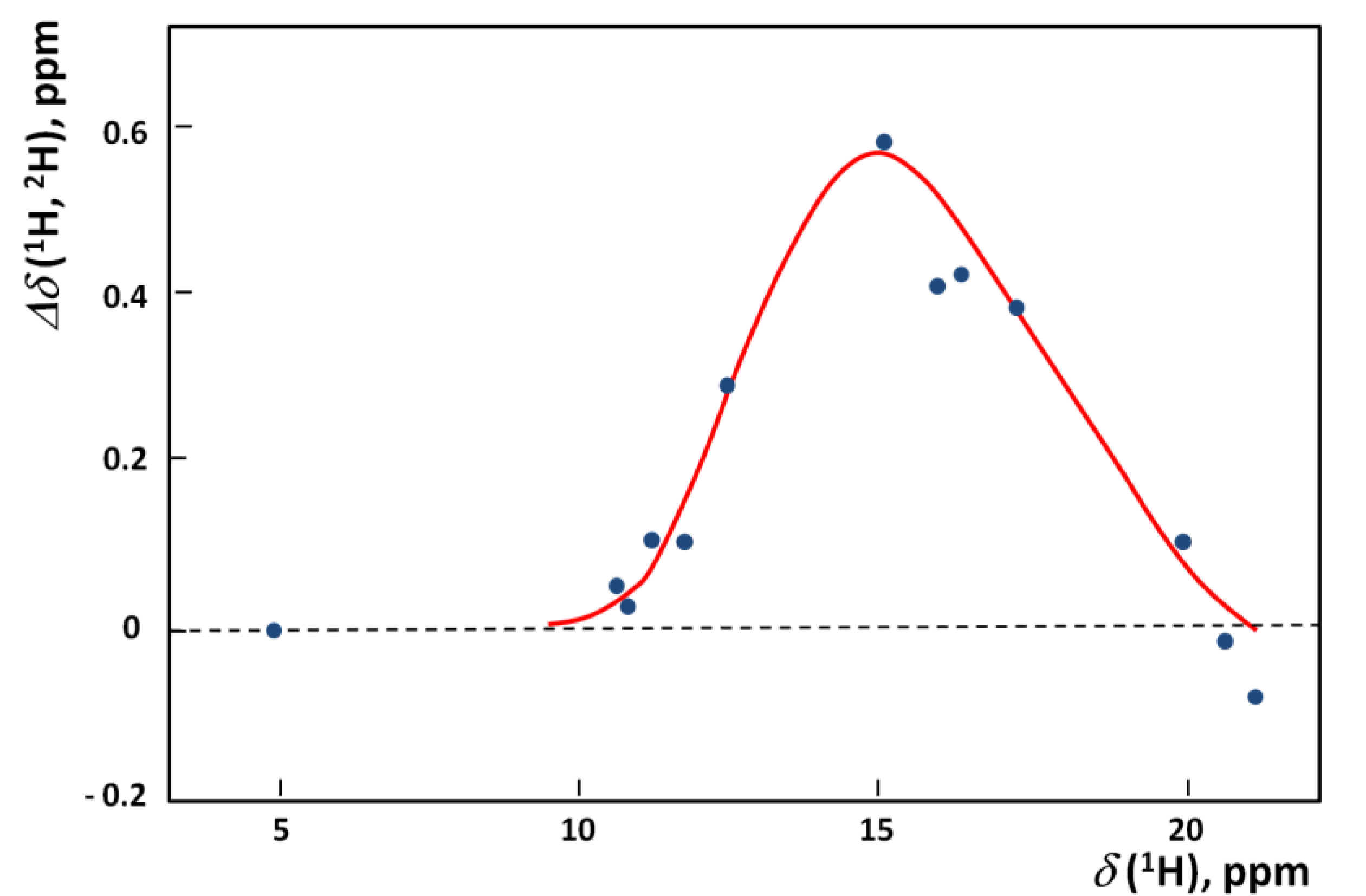
 . When hydrogen bonds are formed this ratio usually undergoes some reduction. When the hydrogen bond is very strong the value of the isotopic ratio (ISR) reaches a minimum close to unity and then starts to jump intensely.
. When hydrogen bonds are formed this ratio usually undergoes some reduction. When the hydrogen bond is very strong the value of the isotopic ratio (ISR) reaches a minimum close to unity and then starts to jump intensely.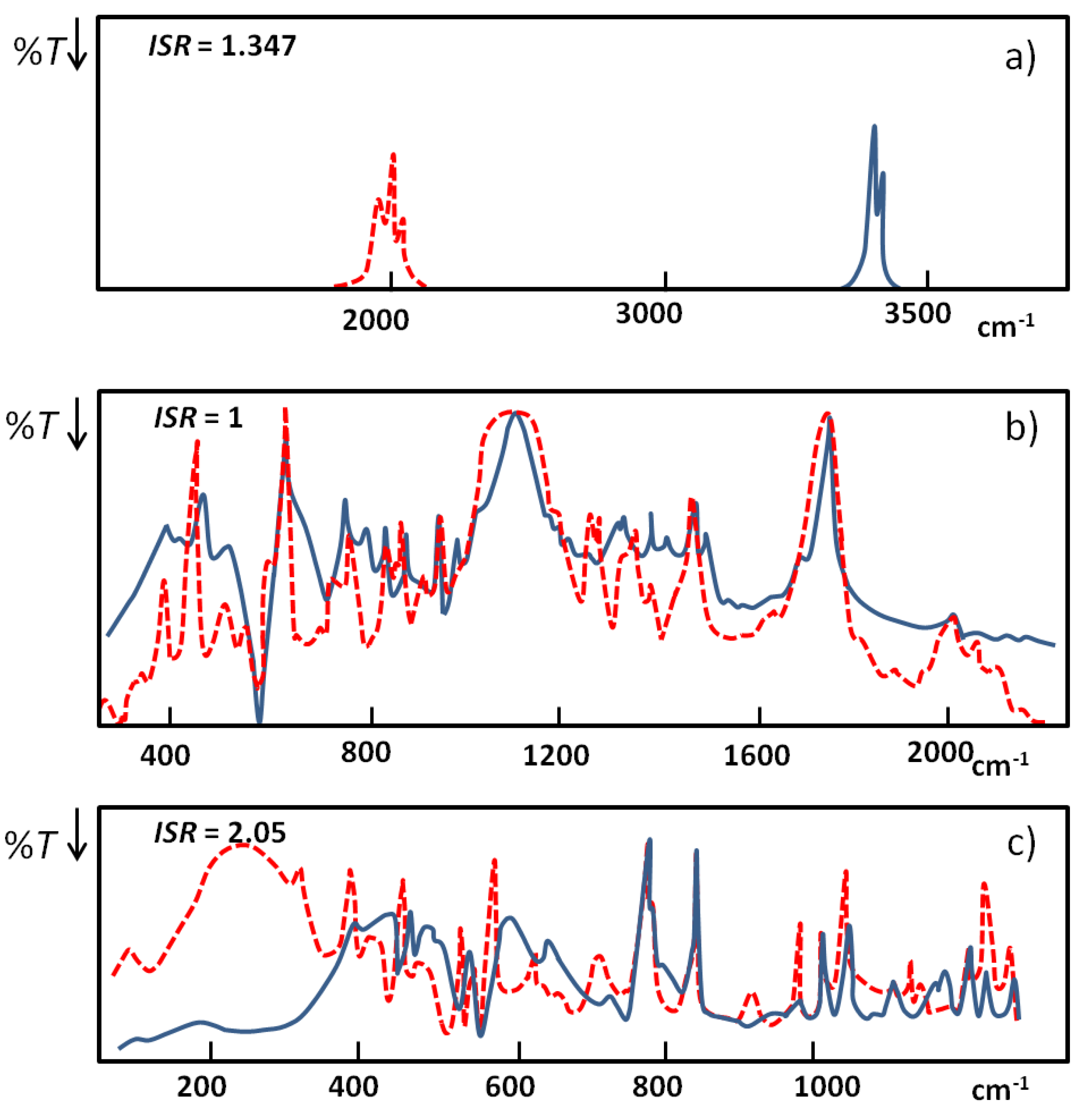
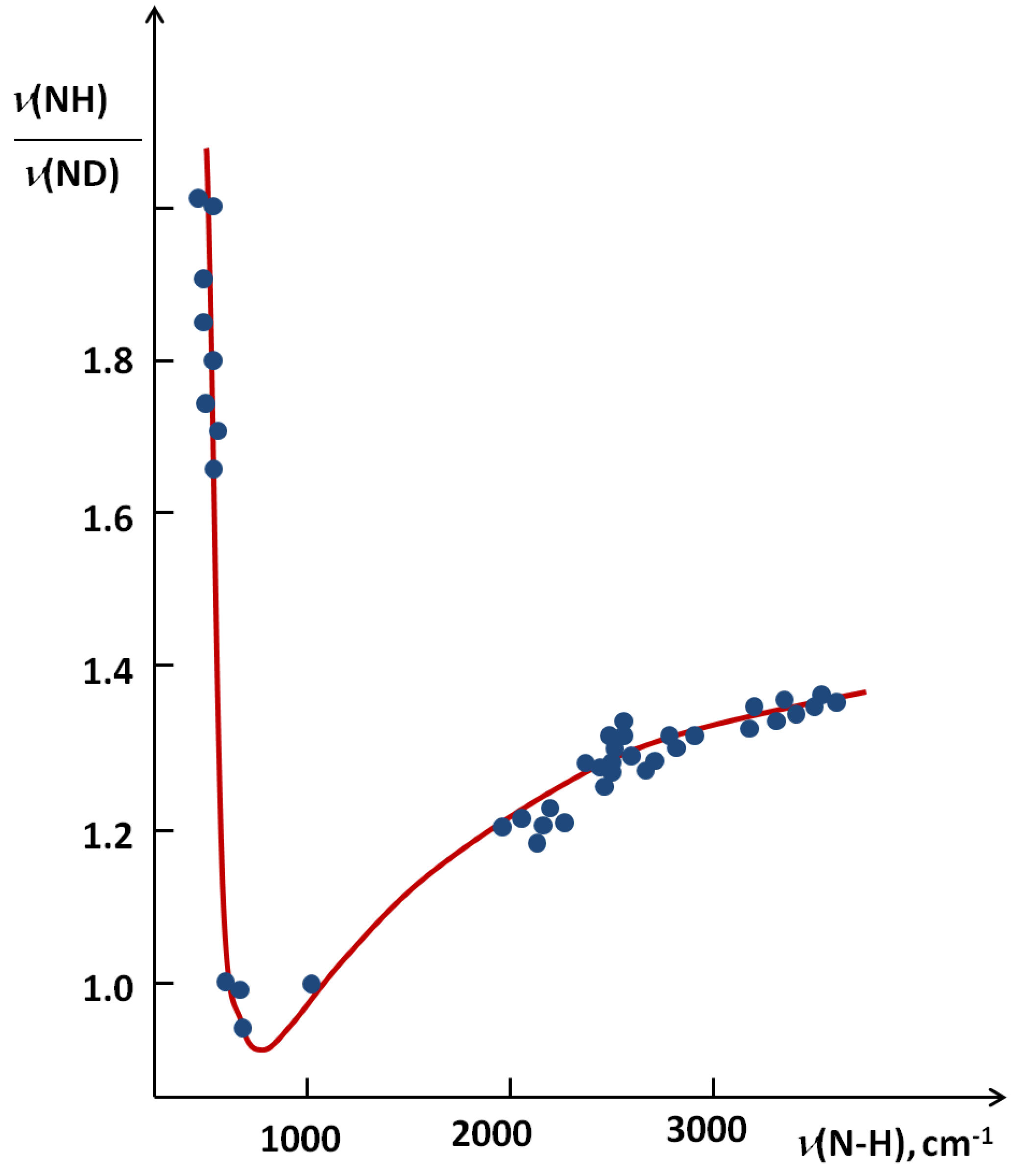

 correspond to a defined critical region which is related for systems with ISR >
correspond to a defined critical region which is related for systems with ISR >  .
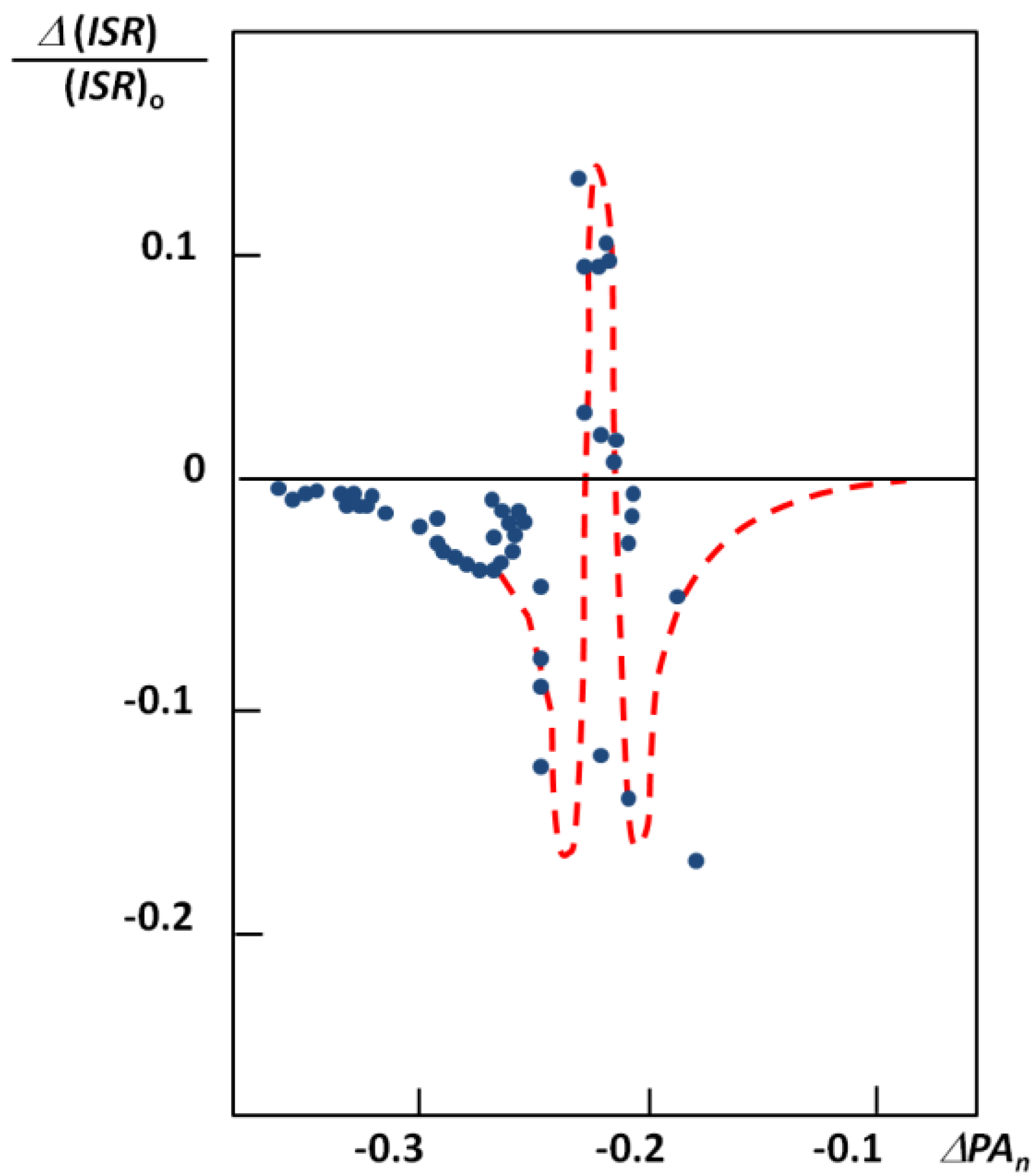
.
3. H/D Isotope Effects in NQR and NMR Spectra
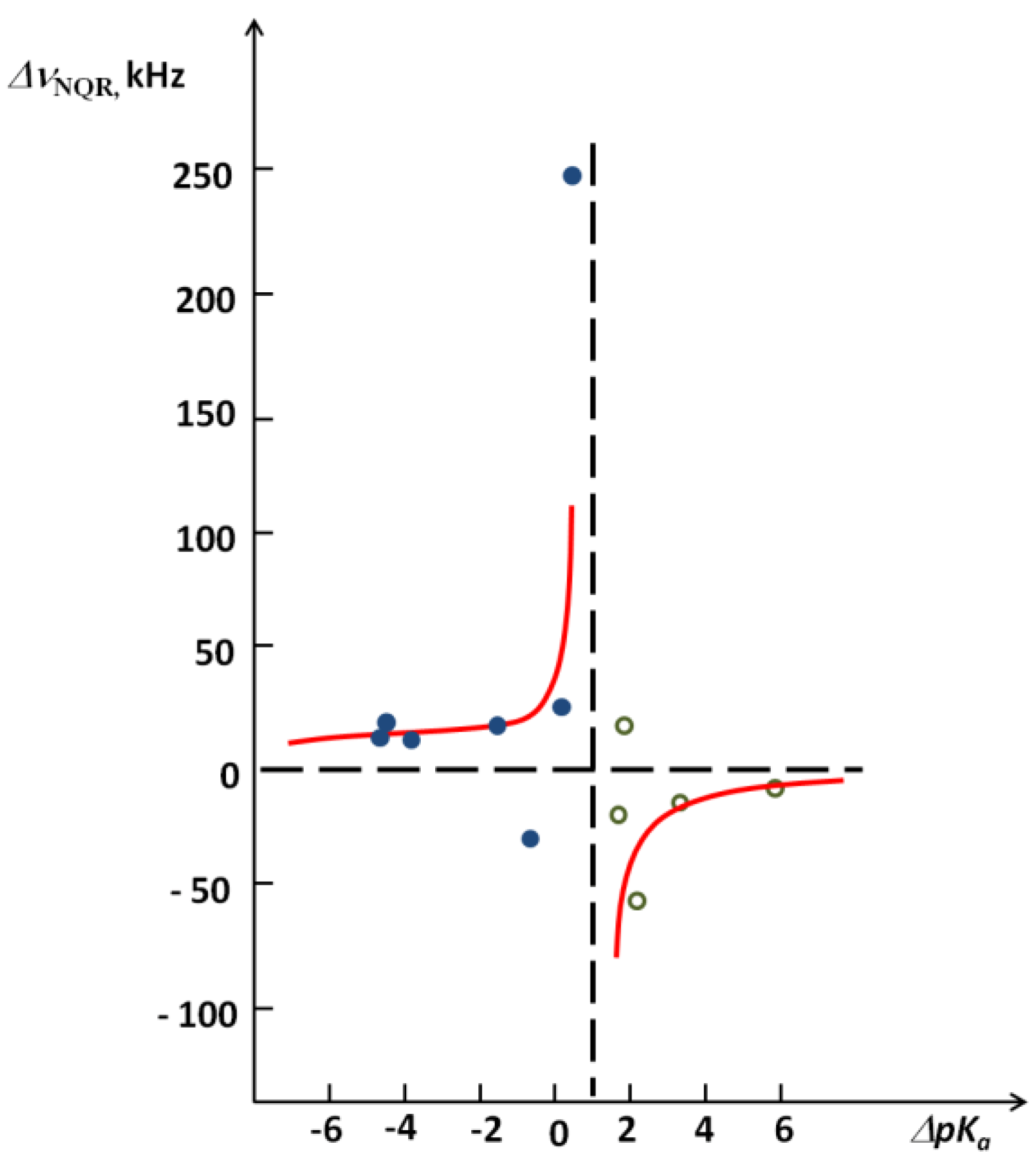
 as shown in Figure 8 [26]. A critical ΔpKa region where a stepwise decrease of the νNQR value takes place is visible.
as shown in Figure 8 [26]. A critical ΔpKa region where a stepwise decrease of the νNQR value takes place is visible.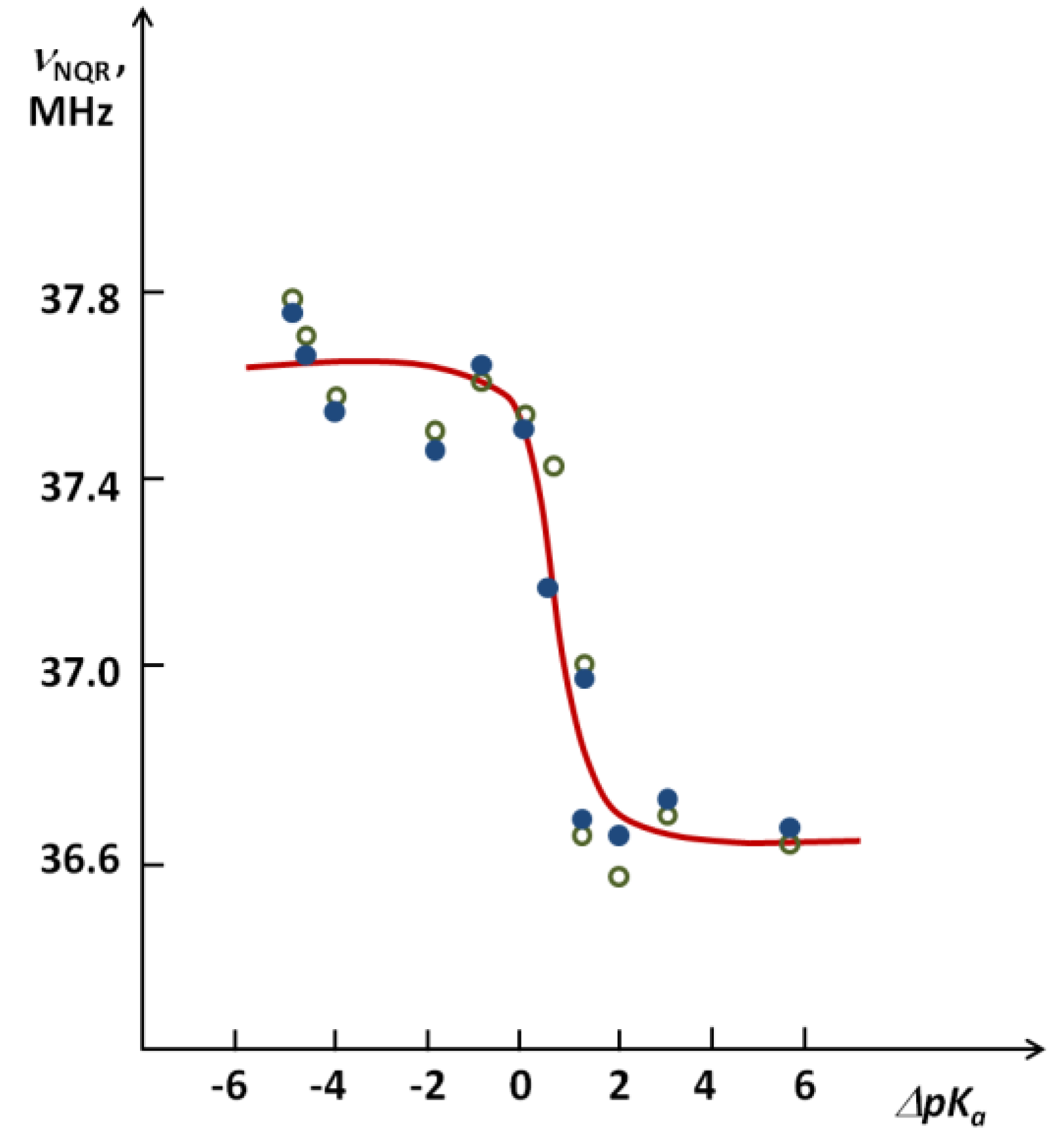
4. Ferroelectric Properties of Hydrogen Bonded Crystals
5. Conclusion
References
- Pimentel, G.C.; McClellan, A.L. The Hydrogen Bond; W.H. Freeman: San Francisco, CA, USA, 1960. [Google Scholar]
- Vinogradov, S.N.; Linnel, R.H. Hydrogen Bonding; Van Nostrand Reinhold: New York, NY, USA, 1971. [Google Scholar]
- Schuster, P.; Zundel, G.; Sandorfy, C. (Eds.) The Hydrogen Bond; North-Holland: Amsterdam, The Netherlands, 1976; Volume 1–3.
- Scheiner, S. Hydrogen Bonding. A Theoretical Perspective; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Hadži, D. (Ed.) Theoretical Treatments of Hydrogen Bonding; John Wiley & Sons: Chichester, UK, 1997.
- Grabowski, S.J. (Ed.) Hydrogen Bonding—New Insights; Springer: Dordrecht, The Netherlands, 2006.
- Gilli, G.; Gilli, P. The Nature of the Hydrogen Bond; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Kohen, A.; Limbach, H.H. (Eds.) Isotope Effects in Chemistry and Biology; Taylor & Francis, CRC Press: Boca Raton, FL, USA, 2006.
- Sobczyk, L. Hydrogen bonding: Selected problems. Wiad. Chem. 2001, 55, 593–627. [Google Scholar]
- Mielke, Z.; Sobczyk, L. Vibrational Isotope Effects in Hydrogen Bonds. In Isotope Effects in Chemistry and Biology; Kohen, A., Limbach, H.H., Eds.; Taylor & Francis, CRC Press: Boca Raton, FL, USA, 2006; pp. 281–304. [Google Scholar]
- Zeegers-Huyskens, T.; Sobczyk, L. Critical behaviour of strong hydrogen bonds and the isotopic effects. J. Mol. Liq. 1990, 46, 263–284. [Google Scholar] [CrossRef]
- Denisov, G.S.; Mavri, J.; Sobczyk, L. Potential energy shape for the proton motion in hydrogen bond reflected in infrared and NMR spectra. In Hydrogen Bonding—New Insights; Grabowski, S.J., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 377–416. [Google Scholar]
- Sobczyk, L.; Czarnik-Matusewicz, B.; Rospenk, M.; Obrzud, M.H. Proton transfer equilibria and critical behavior of H-bonding. J. Atom. Mol. Opt. Phys. 2012, 217932, 1–10. [Google Scholar]
- Huyskens, P.L.; Sobczyk, L.; Majerz, I. On a hard/soft hydrogen bond interaction. J. Mol. Struct. 2002, 615, 61–72. [Google Scholar] [CrossRef]
- Hansen, P.E.; Rozwadowski, Z.; Dziembowska, T. Nuclear magnetic resonance spectroscopy of hydroxy schiff bases. Curr. Org. Chem. 2009, 13, 194–215. [Google Scholar] [CrossRef]
- Perrin, C.L. Symmetry of hydrogen bonds in solution. Pure Appl. Chem. 2009, I, 571–583. [Google Scholar] [CrossRef]
- Limbach, H.-H.; Tolstoy, P.M.; Pérez-Hernández, N.; Guo, J.; Shenderovich, I.G.; Denisov, G.S. OHO hydrogen bond geometries and NMR chemical shifts: From squilibrium structures to geometric H/D isotope effects, With applications for water, protonated water, And compressed ice. Isr. J. Chem. 2009, 49, 199–216. [Google Scholar] [CrossRef]
- Perrin, C.L.; Ohta, B.K. Symmetry of N-H-N Hydrogen Bonds in 1,8-Bis(dimethylamino)-naphthalene H+ and 2,7-Dimethoxy-1,8-bis(dimethylamino)naphthalene H+. J. Am. Chem. Soc. 2001, 123, 6520–6526. [Google Scholar] [CrossRef]
- Ip, B.C.K.; Shenderovich, I.G.; Tolstoy, P.M.; Frydel, J.; Denisov, G.S.; Buntkowsky, G.; Limbach, H.-H. NMR Studies of Solid Pentachlorophenol-4-Methylpyridine Complexes Exhibiting Strong OHN Hydrogen Bonds: Geometric H/D Isotope Effects and Hydrogen Bond Coupling Cause Isotopic Polymorphism. J. Phys. Chem. A 2012, 116, 11370–11387. [Google Scholar] [CrossRef]
- O´Leary, D.J.; Hickstein, D.D.; Hansen, B.K.V.; Hansen, P.E. Theoretical and NMR studies of deuterium isotopic perturbation of hydrogen bonding in symmetrical dihydroxy compounds. J. Org. Chem. 2010, 75, 1331–1342. [Google Scholar]
- Matsushita, E.; Matsubara, T. Note on isotope effect in hydrogen bonded crystals. Prog. Theor. Phys. 1982, 67, 1–19. [Google Scholar] [CrossRef]
- Sobczyk, L. On a “Critical” Behaviour of Strong Hydrogen Bonds. In Interactions of Water in Ionic and Nonionic Hydrates. Proceedings of a Symposium in Honour of the 65th Birthday of W.A.P. Luck Marbury/FRG, 2.–3.4. 1987; Springer Berlin Heidelberg: Berlin/Heidelbery, Germany, 1987; pp. 283–286. [Google Scholar]
- Grech, E.; Malarski, Z.; Sobczyk, L. Isotopic effects in nitrogen-hydrogen...nitrogen (NH…N) hydrogen bonds. Chem. Phys. Lett. 1986, 128, 259–263. [Google Scholar] [CrossRef]
- Zeegers-Huyskens, T.; Sobczyk, L. Isotopic ratio νHX/νDX of hydrogen halide complexes in solid argon. Spectrochim. Acta A 1990, 46A, 1693–1698. [Google Scholar] [CrossRef]
- Kalenik, J.; Majerz, I.; Malarski, Z.; Sobczyk, L. Isotopic effect on 35Cl NQR spectra of pentachlorophenol-amine hydrogen-bonded complexes. Chem. Phys. Lett. 1990, 165, 15–18. [Google Scholar] [CrossRef]
- Gunnarsson, G.; Wennerstrom, H.; Egan, W.; Forsen, S. Proton and deuterium NMR of hydrogen bonds: relationship between isotope effects and the hydrogen bond potential. Chem. Phys. Lett. 1976, 38, 96–99. [Google Scholar] [CrossRef]
- Ichikawa, M. The O-H vs O…O distance correlation, the geometric isotope effect in OHO bonds, And its application to symmetric bonds. Acta Cryst 1978, B34, 2074–2080. [Google Scholar]
- Ichikawa, M. Correlation between two isotope effects in hydrogen-bonded crystals: Transition temperature and separation of two equilibrium sites. Chem. Phys. Lett. 1981, 79, 583–587. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sobczyk, L.; Obrzud, M.; Filarowski, A. H/D Isotope Effects in Hydrogen Bonded Systems. Molecules 2013, 18, 4467-4476. https://doi.org/10.3390/molecules18044467
Sobczyk L, Obrzud M, Filarowski A. H/D Isotope Effects in Hydrogen Bonded Systems. Molecules. 2013; 18(4):4467-4476. https://doi.org/10.3390/molecules18044467
Chicago/Turabian StyleSobczyk, Lucjan, Monika Obrzud, and Aleksander Filarowski. 2013. "H/D Isotope Effects in Hydrogen Bonded Systems" Molecules 18, no. 4: 4467-4476. https://doi.org/10.3390/molecules18044467




