Potential Energy Surfaces and Quantum Yields for Photochromic Diarylethene Reactions
Abstract
:1. Introduction
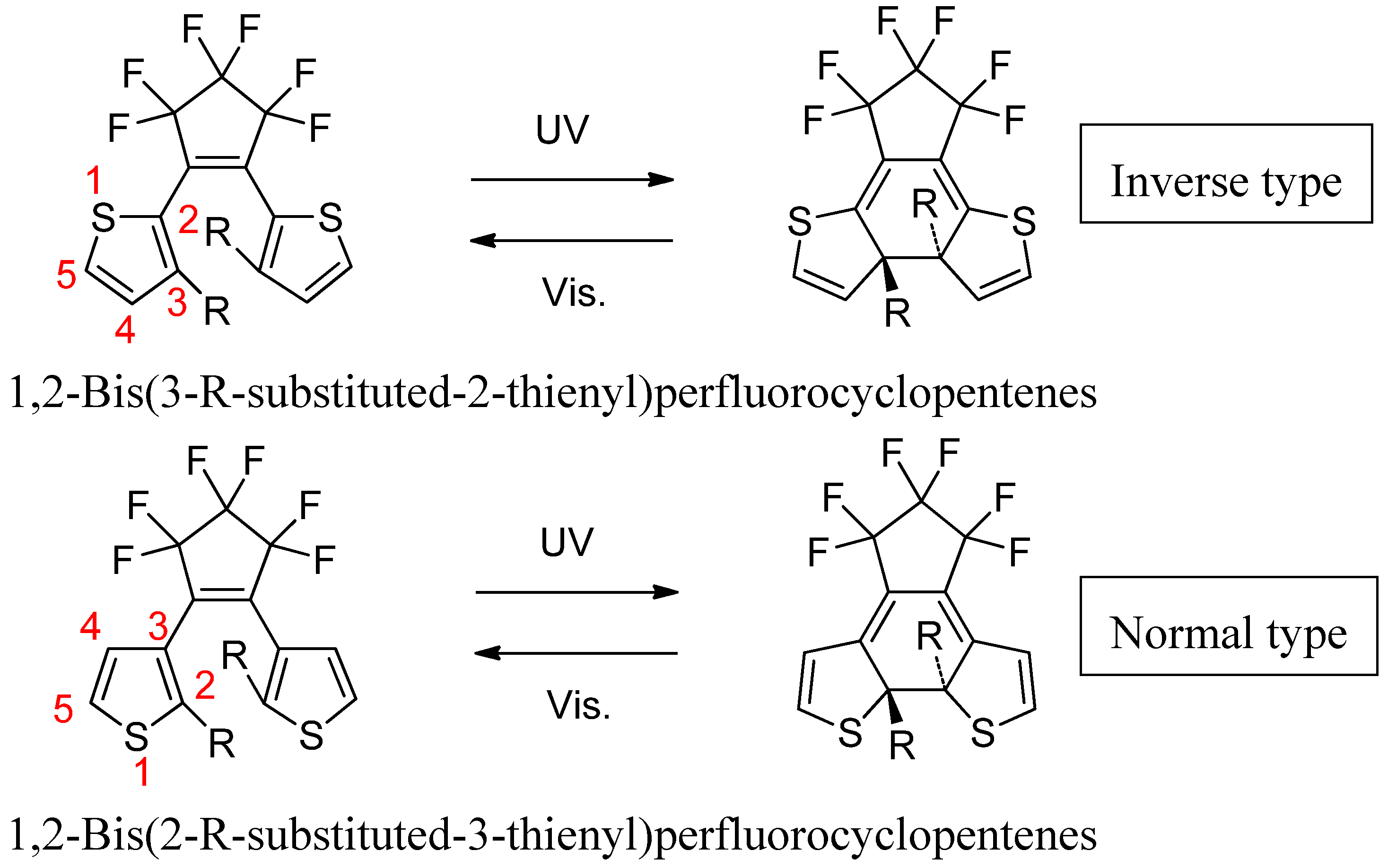
2. Two Categories of DAE Molecules
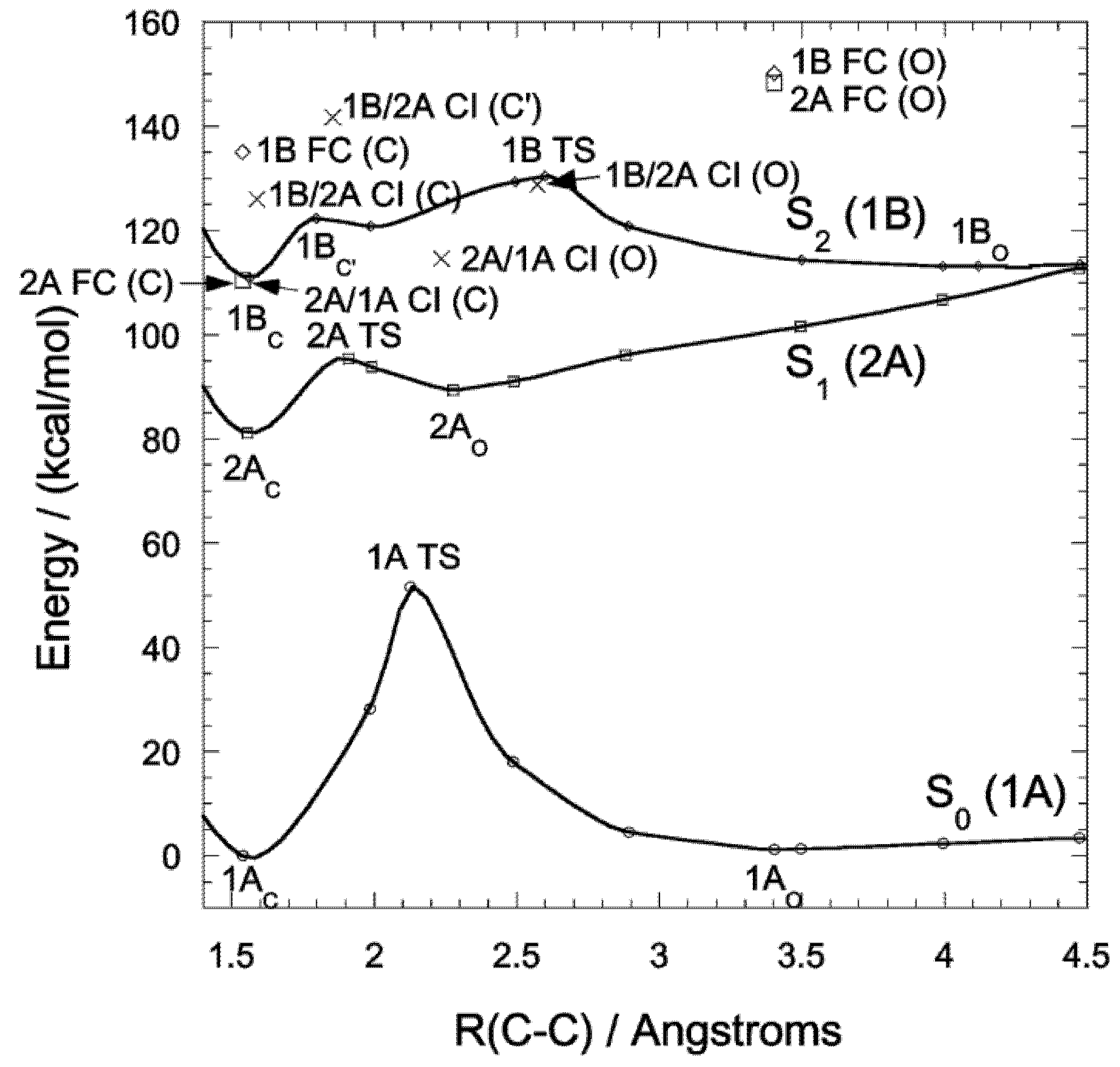
3. DAE Photochromism Mechanism Inferred from the Potential Energy Surfaces
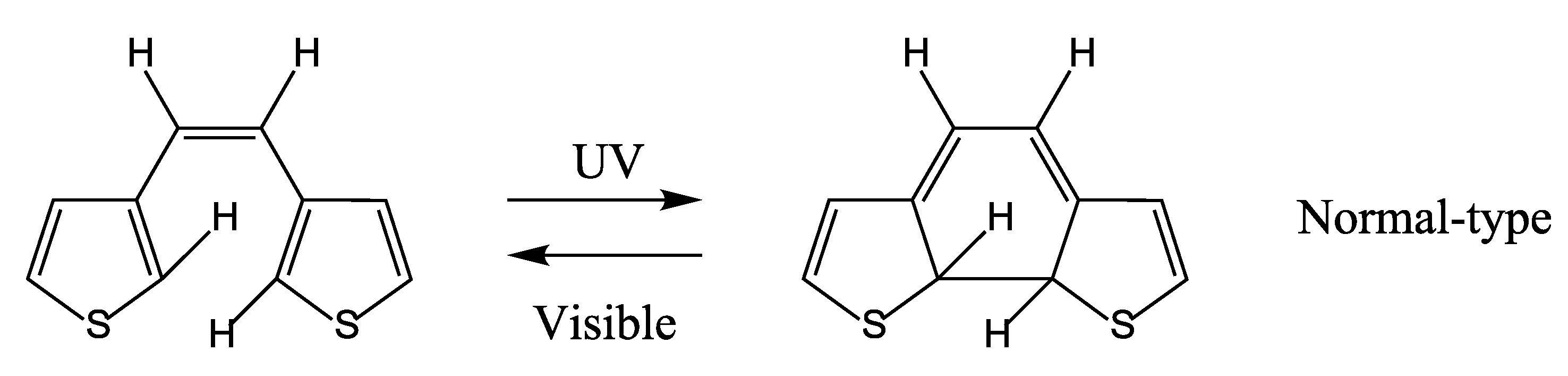
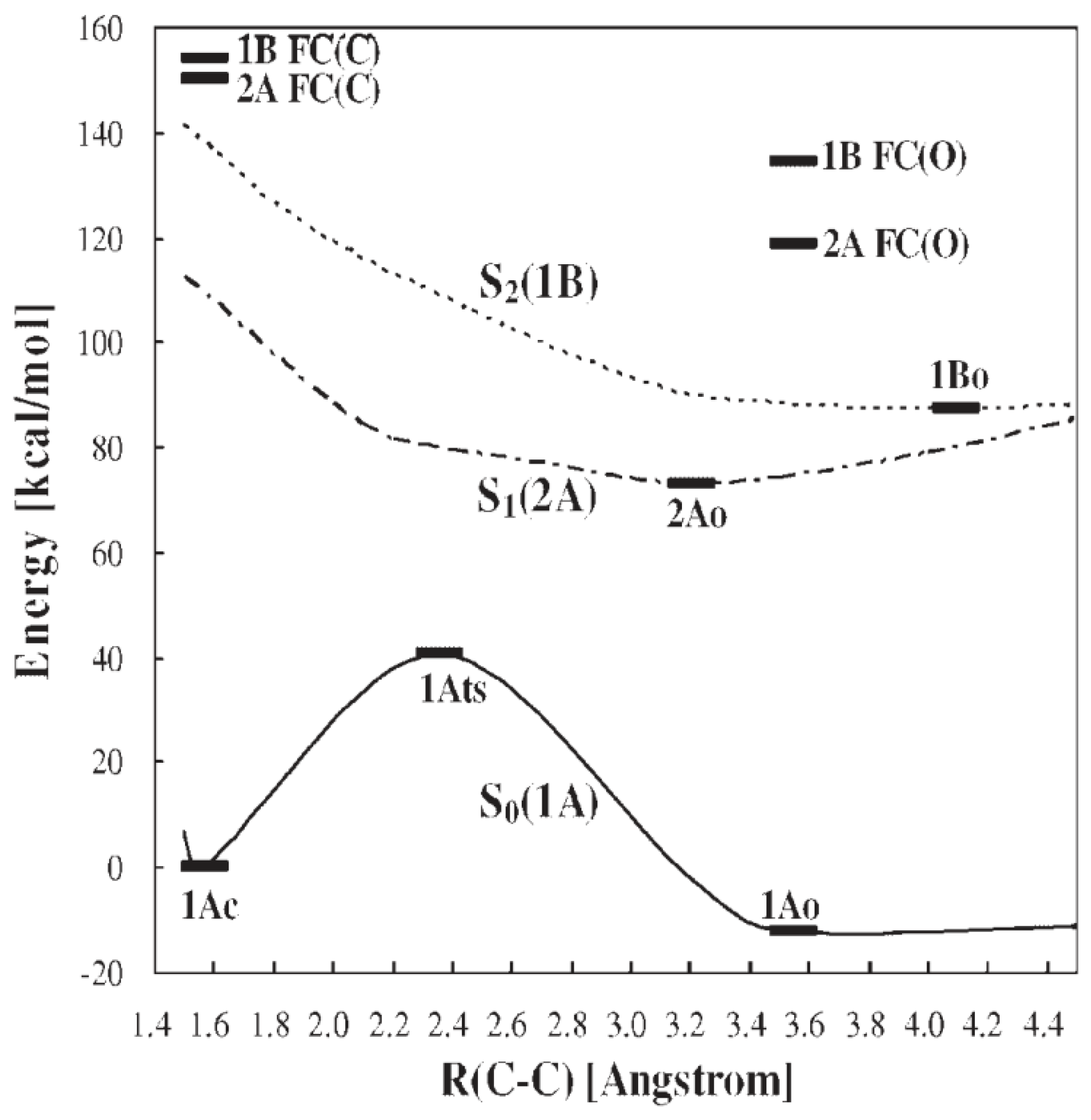
4. QYs for Normal-Type DAEs
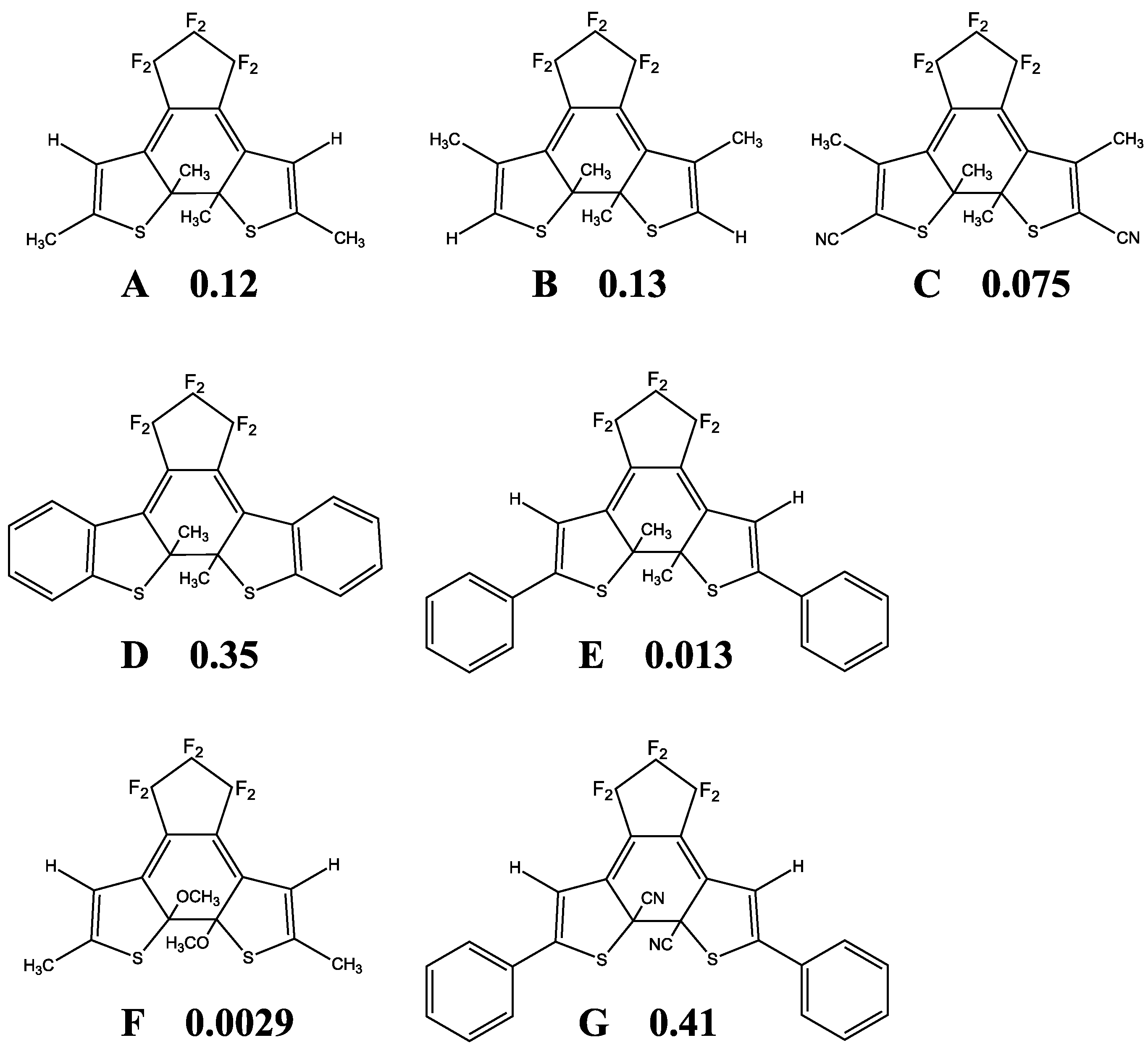
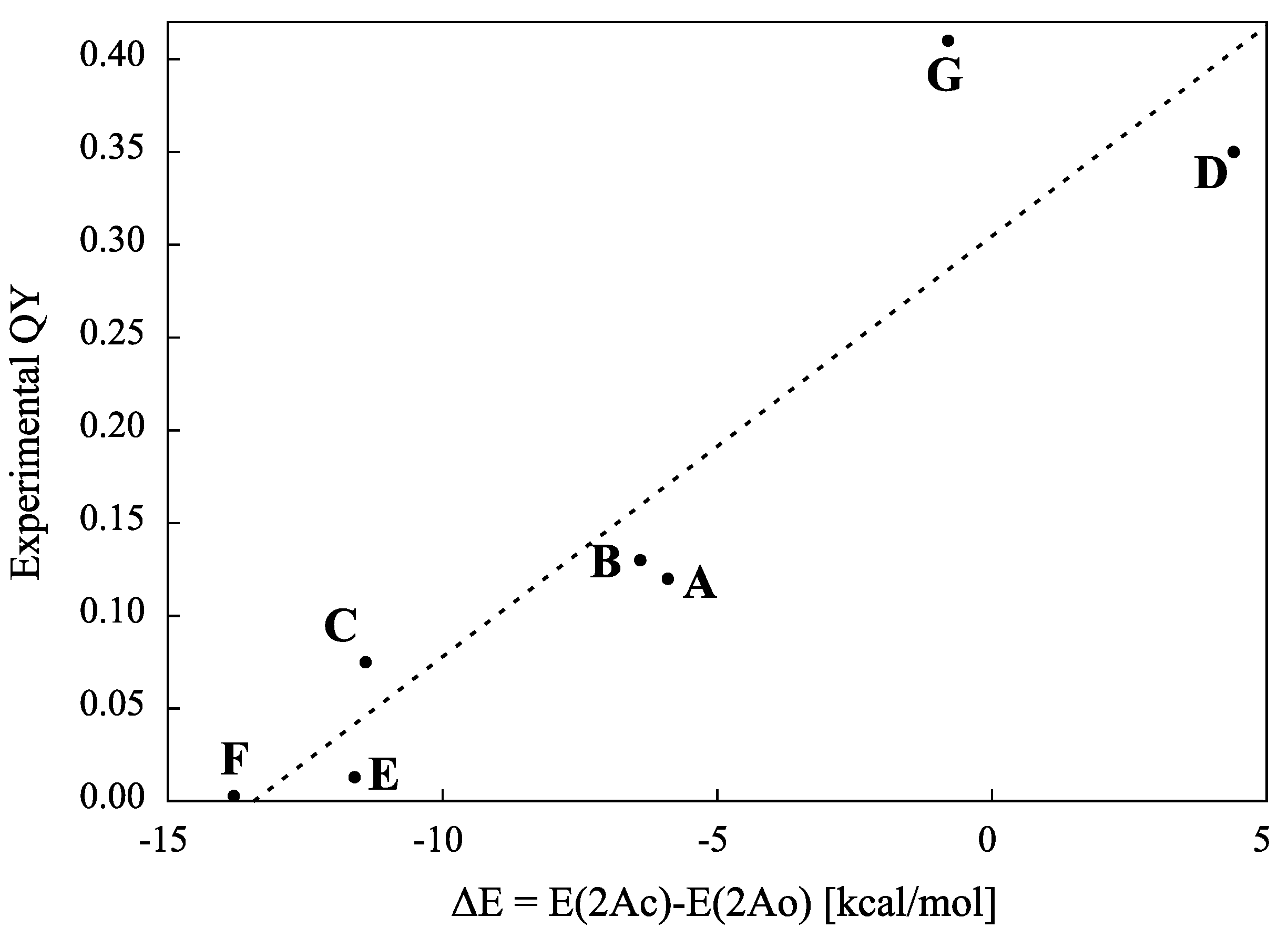
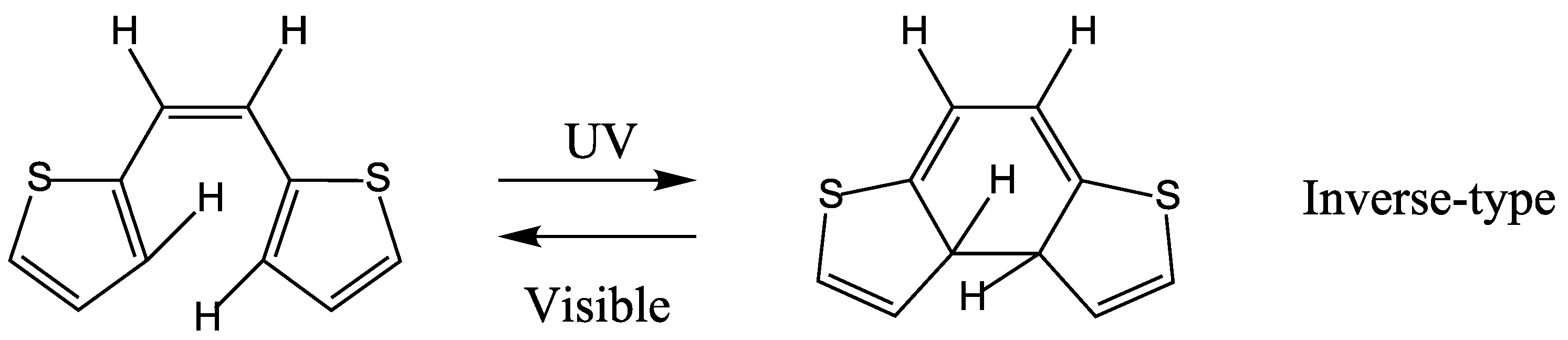
5. QYs for Inverse-Type DAEs
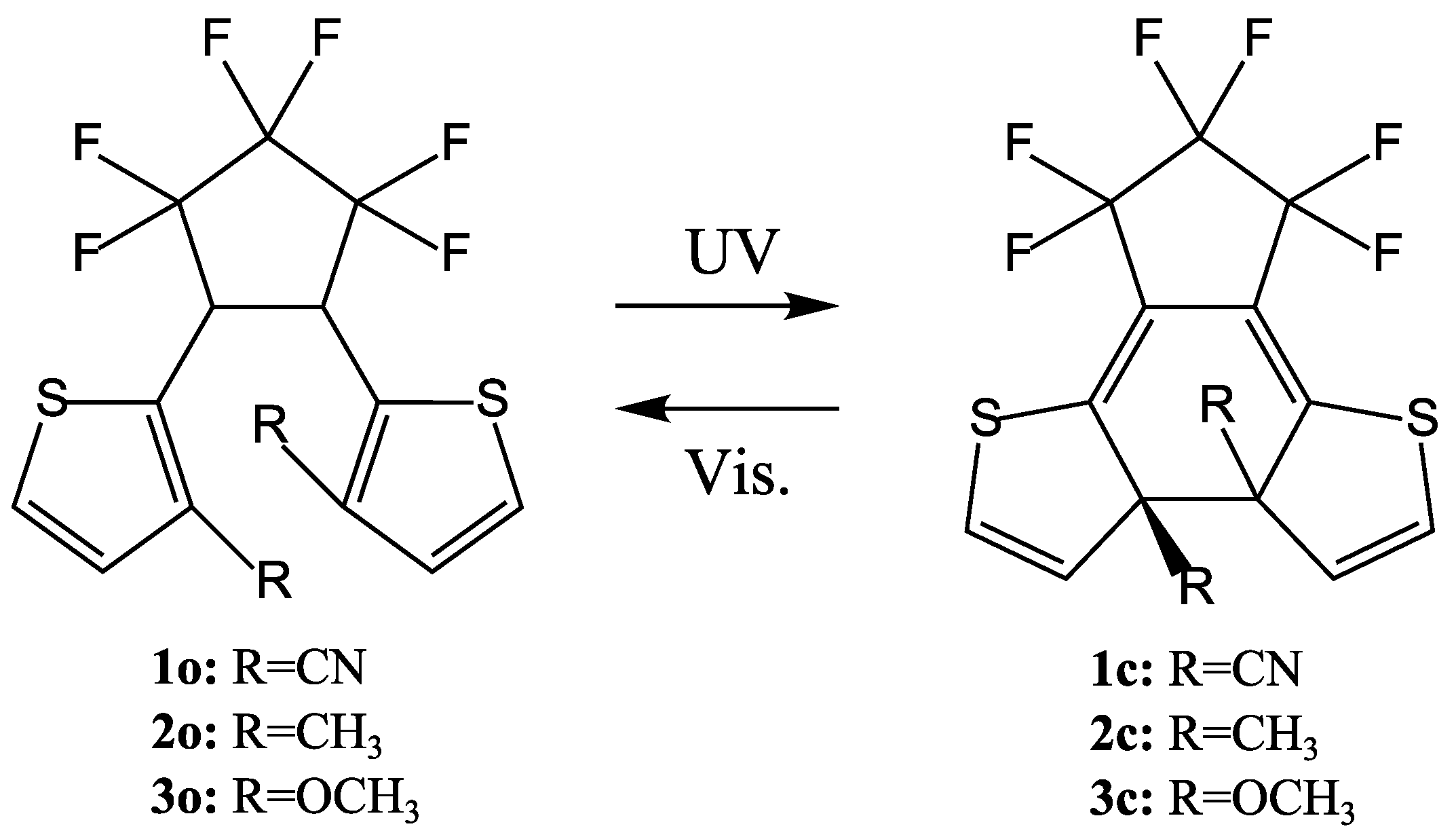
| λmax/nm (ε/M−1 cm−1) | Φoc | λmax/nm (ε/M−1 cm−1) | Φco | ||
|---|---|---|---|---|---|
| 1o | 258 (1.27 × 104) | 0.17 (366 nm) | 1c | 427 (9.1 × 103) | 0.45 (435 nm) |
| 289 (1.30 × 104) | |||||
| 236 (1.24 × 104) | |||||
| 2o | 319 (1.55 × 104) | 0.25 (313 nm) | 2c | 432 (8.8 × 103) | 0.37 (435 nm) |
| 3o | 327 (1.8 × 104) | 0.22 (366 nm) | 3c | 481 (5.8 × 103) | 0.25 (435 nm) |
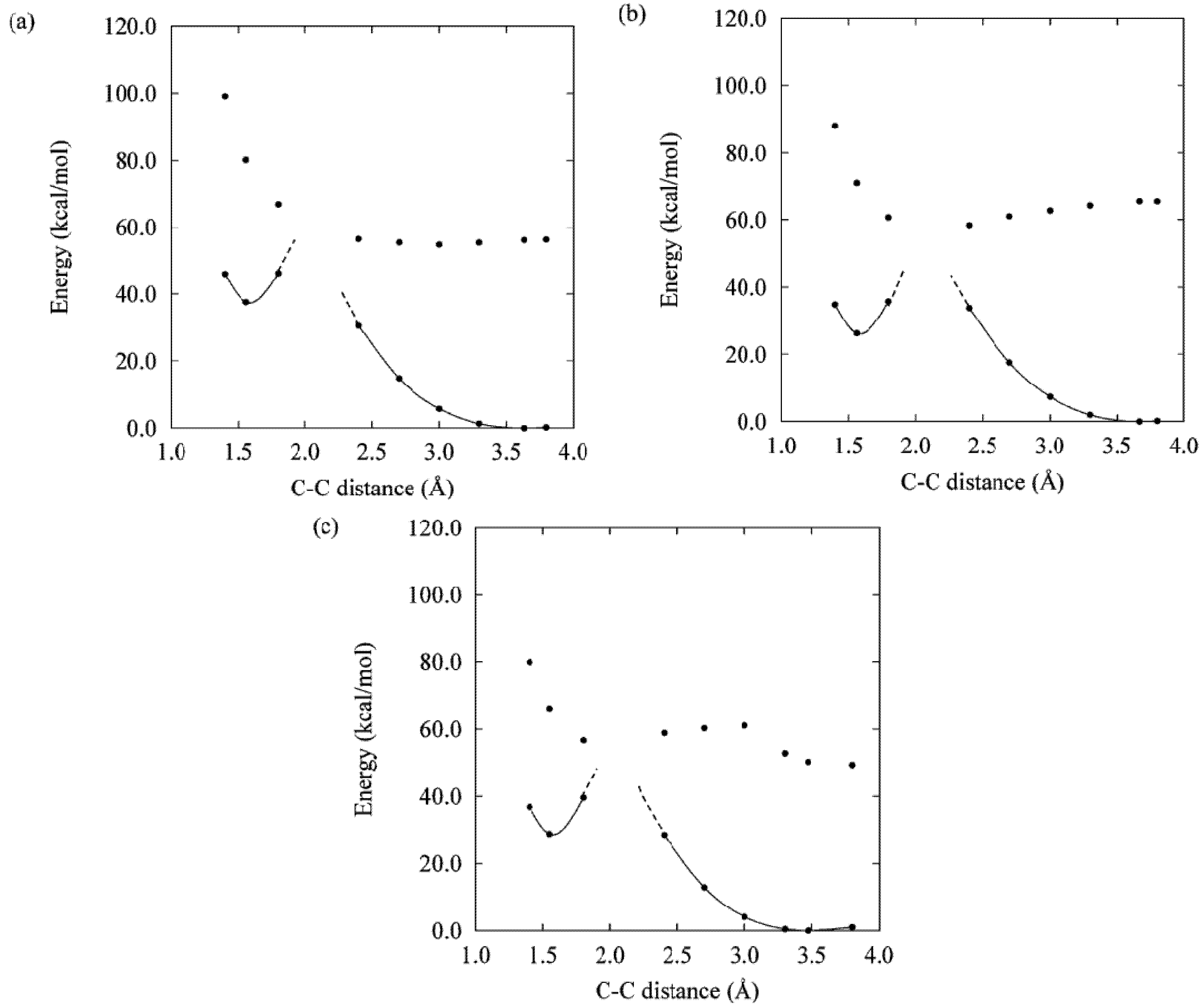
6. Two PES Profiles and Remaining QY-Design Problems
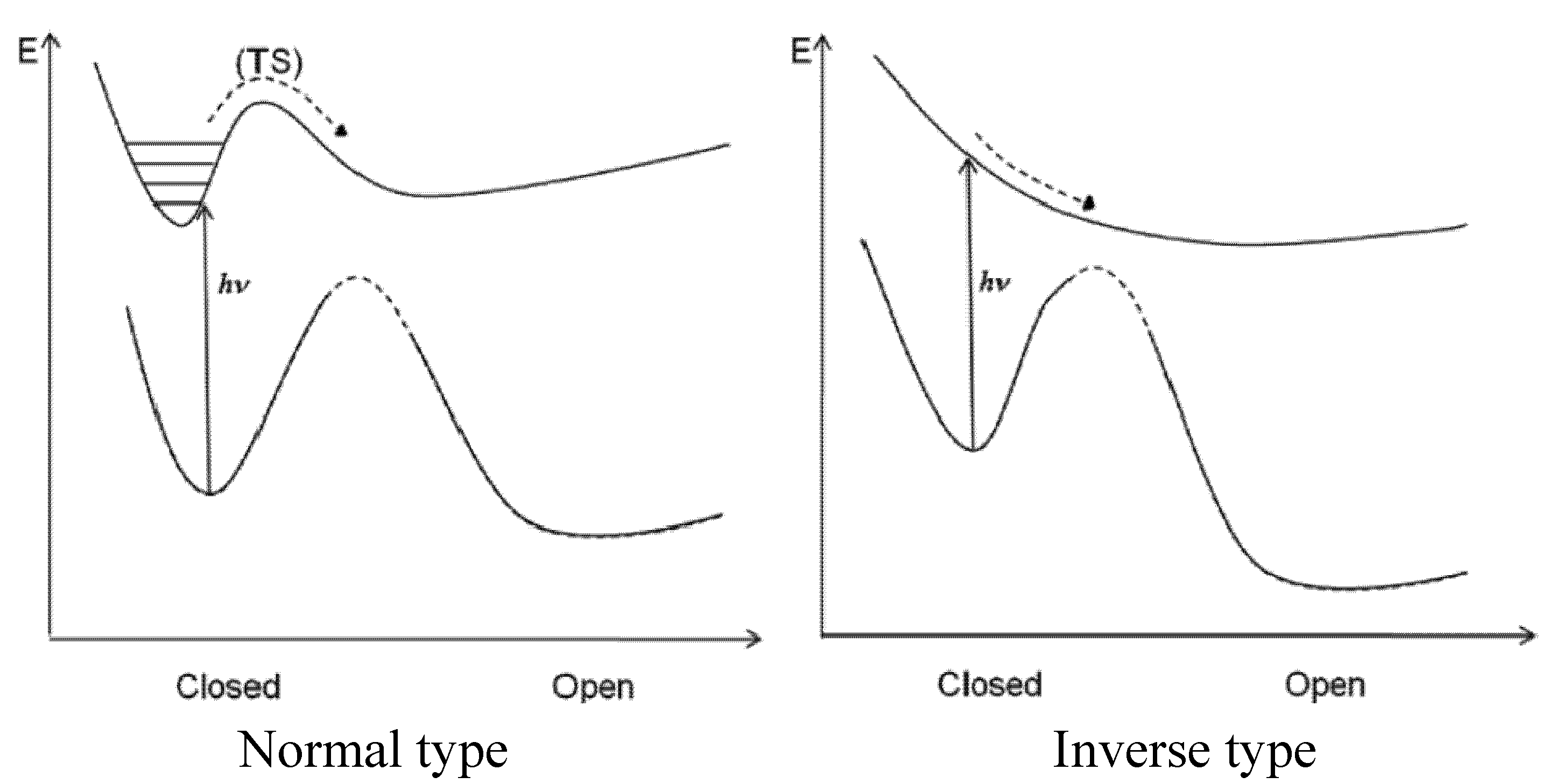

7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Crano, J.C.; Guglielmetti, R.J. (Eds.) Organic Photochromic and Thermochromic Compounds Volume 1 Main Photochromic Families; Plenum: New York, NY, USA, 1999.
- Crano, J.C.; Guglielmetti, R.J. (Eds.) Organic Photochromic and Thermochromic Compounds, Volume 2 Physicochemical Studies, Biological Applications, and Thermochromism; Plenum: New York, NY, USA, 1999.
- Irie, M. Diarylethenes for memories and switches. Chem. Rev. 2000, 100, 1685–1716. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X.; Willner, I. Integration of photoswitchable proteins, photosynthetic reaction centers and semiconductor/biomolecule hybrids with electrode supports for optobioelectronics applications. Adv. Mater. 2013, 25, 349–377. [Google Scholar] [CrossRef]
- Nakamura, S.; Irie, M. Thermally irreversible photochromic systems. A theoretical study. J. Org. Chem. 1988, 53, 6136–6138. [Google Scholar] [CrossRef]
- Uchida, K.; Nakamura, S.; Irie, M. Photochromism of dinaphthylethene derivatives. Stability of the closed-ring forms. Res. Chem. lntermed. 1995, 21, 861–876. [Google Scholar] [CrossRef]
- Uchida, K.; Nakamura, S.; Irie, M. Thermally irreversible photochromic systems. Substituent effect on the absorption wavelength of 11,12-dicyano-5a, 5b-dihydro-5a,5b-dimethylbenzo[1,2-b:6,5-b']bis[1]benzothiophene. Bull. Chem. Soc. Jpn. 1992, 65, 430–435. [Google Scholar] [CrossRef]
- Nakamura, S.; Kobayashi, T.; Takata, A.; Uchida, K.; Asano, Y.; Murakami, A.; Goldberg, A.; Guillaumont, D.; Yokojima, S.; Kobatake, S.; Irie, M. Quantum Yields and potential energy surfaces: A theoretical study. J. Phys. Org. Chem. 2007, 20, 821–829. [Google Scholar] [CrossRef]
- Goldberg, A.; Murakami, A.; Kanda, K.; Kobayashi, T.; Nakamura, S.; Uchida, K.; Sekiya, H.; Fukaminato, T.; Kawai, T.; Kobatake, S.; Irie, M. Rotational isomerization of dithienylethenes: A study on the mechanism determining quantum yield of cyclization reaction. J. Phys. Chem. A 2003, 107, 4982–4988. [Google Scholar] [CrossRef]
- Uchida, K.; Tsuchida, E.; Aoi, Y.; Nakamura, S.; Irie, M. Substitution effect on the coloration quantum yield of a photochromic bisbenzothienylethene. Chem. Lett. 1999, 28, 63–64. [Google Scholar]
- Okabe, C.; Tanaka, N.; Fukaminato, T.; Kawai, T.; Irie, M.; Nibu, Y.; Shimada, H.; Goldberg, A.; Nakamura, S.; Sekiya, H. Raman spectroscopic study on photochromic reaction of a diarylethene derivative. Chem. Phys. Lett. 2002, 357, 113–118. [Google Scholar] [CrossRef]
- Uchida, K.; Saito, M.; Murakami, A.; Nakamura, S.; Irie, M. Non-destructive readout of the photochromic reactions of diarylethene derivatives using infra-red light. Adv. Mater. 2003, 15, 121–125. [Google Scholar] [CrossRef]
- Takata, A.; Yokojima, S.; Nakagawa, H.; Matsuzawa, Y.; Murakami, A.; Nakamura, S.; Irie, M.; Uchida, K. Substituent effect of diarylethenes on IR spectra for application of non-destructive readout of photochromic recording. J. Phys. Org. Chem. 2007, 20, 998–1006. [Google Scholar] [CrossRef]
- Takata, A.; Saito, M.; Yokojima, S.; Murakami, A.; Nakamura, S.; Irie, M.; Uchida, K. Micrometer-scale photochromic recording on an amorphous diarylethene film and nondestructive readout using near-field IR light. Jpn. J. Appl. Phys. 2006, 45, 7114–7120. [Google Scholar] [CrossRef]
- Uchida, K.; Saito, M.; Murakami, A.; Kobayashi, T.; Nakamura, S.; Irie, M. Three bits eight states photochromic recording and nondestructive readout by using IR light. Chem. Eur. J. 2005, 11, 534–542. [Google Scholar] [CrossRef]
- Matsuda, K.; Yokojima, S.; Moriyama, Y.; Nakamura, S.; Irie, M. Direct observation of cation radicals of a diarylethene during oxidative ring-opening reaction. Chem. Lett. 2006, 35, 900–901. [Google Scholar] [CrossRef]
- Yokojima, S.; Matsuda, K.; Irie, M.; Murakami, A.; Kobayashi, T.; Nakamura, S. Characterization of cationic diarylethene by electron spin resonance and absorption spectra-ratio of open/closed-ring isomers. J. Phys. Chem. A 2006, 110, 8137–8143. [Google Scholar] [CrossRef]
- Tsujioka, T.; Iefuji, N.; Jiapaer, A.; Irie, M.; Nakamura, S. Hole-injection isomerizatio of photochromic diarylethene for organic molecular memory. Appl. Phys. Lett. 2006, 89. [Google Scholar] [CrossRef]
- Fukaminato, T.; Umemoto, T.; Iwata, Y.; Yokojima, S.; Yoneyama, M.; Nakamura, S.; Irie, M. Photochromism of diarylethene single molecules in polymer matrices. J. Am. Chem. Soc. 2007, 129, 5932–5938. [Google Scholar]
- Mikami, M.; Nakamura, S. First-principles study of salicylideneaniline molecular crystals: Tautomerization reaction involving intermolecular hydrogen bonds. Phys. Rev. B 2004, 69. [Google Scholar] [CrossRef]
- Miyasaka, H.; Murakami, M.; Itaya, A.; Guillaumont, D.; Nakamura, S.; Irie, M. Multiphoton gated photochromic reaction in a diarylethene derivative. J. Am. Chem. Soc. 2001, 123, 753–754. [Google Scholar] [CrossRef]
- Irie, M.; Sakemura, K.; Okinaka, M.; Uchida, K. Photochromism of Dithienylethenes with Electron-Donating Substituents. J. Org. Chem. 1995, 60, 8305–8309. [Google Scholar] [CrossRef]
- Morimitsu, K.; Shibata, K.; Kobatake, S.; Irie, M. Dithienylethenes with a novel photochromic performance. J. Org. Chem. 2002, 67, 4574–4578. [Google Scholar] [CrossRef]
- Irie, S.; Irie, M. Radiation-induced coloration of photochromic dithienylethene derivatives in polymer matrice. Bull. Chem. Soc. Jpn. 2000, 73, 2385–2388. [Google Scholar] [CrossRef]
- Morimitsu, K.; Kobatake, S.; Irie, M. Control of cycloreversion quantum yields of diarylethenes by introduction of substituents at the reactive carbons. Mol. Cryst. Liq. Cryst. 2005, 431, 151–154. [Google Scholar]
- Morimitsu, K.; Kobatake, S.; Nakamura, S.; Irie, M. Efficient photocycloreversion reaction of diarylethenes by introduction of cyano subsutituents to the reactive carbon. Chem. Lett. 2003, 32, 858–859. [Google Scholar] [CrossRef]
- Kobatake, S.; Irie, M. Synthesis and photochromism of diarylethenes with isopropyl groups at the reactive carbons and long π-conjugated heteroaryl groups. Chem. Lett. 2003, 32, 1078–1079. [Google Scholar] [CrossRef]
- Uchida, K.; Irie, M. A Photochromic dithienylethene that turns yellow by UV irradiation. Chem. Lett. 1995, 24, 969–970. [Google Scholar] [CrossRef]
- Woodward, R.B.; Hoffmann, R. The Conseruation of Orbital Symmetry; Verlag Chemie, GmbH: New York, NY, USA, 1970. [Google Scholar]
- Guillaumont, D.; Kobayashi, T.; Kanda, K.; Miyasaka, H.; Uchida, K.; Kobatake, S.; Shibata, K.; Nakamura, S.; Irie, M. An ab initio MO study of the photochromic reaction of dithienylethenes. J. Phys. Chem. A 2002, 106, 7222–7227. [Google Scholar] [CrossRef]
- Asano, Y.; Murakami, A.; Kobayashi, T.; Goldberg, A.; Guillaumont, D.; Yabushita, S.; Irie, M.; Nakamura, S. Theoretical study on the photochromic cycloreversion reactions of dithienylethenes; On the role of the conical intersections. J. Am. Chem. Soc. 2004, 126, 12112–12120. [Google Scholar] [CrossRef]
- Kobatake, S.; Uchida, K.; Tsuchida, E.; Irie, M. Single-crystalline photochromism of diarylethenes: reactivity–structure relationship. Chem. Commun. 2002, 2804–2805. [Google Scholar]
- Shibata, K.; Muto, K.; Kobatake, S.; Irie, M. Photocyclization/cycloreversion quantum yields of diarylethenes in single crystals. J. Phys. Chem. A 2002, 106, 209–214. [Google Scholar] [CrossRef]
- Morinaka, K.; Ubukata, T.; Yokoyama, Y. Structurally versatile novel photochromic bisarylindenone and its acetal: Achievement of large cyclization quantum yield. Org. Lett. 2009, 11, 3890–3893. [Google Scholar] [CrossRef]
- Tatsumi, Y.; Kitai, J.; Uchida, W.; Ogata, K.; Nakamura, S.; Uchida, K. Photochromism of 1,2-bis(2-thienyl)perfluorocyclopentene derivatives: Substituent effect on the reactive carbon atoms. J. Phys. Chem. A 2012, 116, 10973–10979. [Google Scholar]
- Kitai, J.; Kobayashi, T.; Uchida, W.; Hatakeyama, M.; Yokojima, S.; Nakamura, S.; Uchida, K. Photochromism of a diarylethene having an azulene ring. J. Org. Chem. 2012, 77, 3270–3276. [Google Scholar] [CrossRef]
- Irie, M.; Mohri, M. Thermally irreversible photochromic systmes. Reversible photocyclization of diaryleethene derivatives. J. Org.Chem. 1988, 53, 803–808. [Google Scholar] [CrossRef]
- Irie, M.; Seki, T.; Yokoyama, Y. (Eds.) New Frontiers in Photochromism; Springer: Tokyo, Japan, 2013.
- Ern, J.; Bens, A.T.; Martin, H.D.; Mukamel, S.; Schmid, D.; Tretiak, S.; Tsiper, E.; Kryschi, C. Reaction dynamics of a photochromic fluorescing dithienylethene. J. Phys. Chem. A 2001, 105, 1741–1749. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Okuno, K.; Ota, C.; Umesato, T.; Katayama, T.; Murakami, M.; Kobatake, S.; Irie, M.; Miyasaka, H. Multiphoton-gated cycloreversion reactions of photochromic diarylethene derivatives with low reaction yields upon one-photon visible excitation. Photochem. Photobiol. Sci. 2010, 9, 172–180. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Umesato, T.; Kobatake, S.; Irie, M.; Miyasaka, H. Femtosecond laser photolysis studies on temperature dependence of cyclization and cycloreversion reactions of a photochromic diarylethene derivative. J. Phys. Chem. C 2012, 116, 4862–4859. [Google Scholar]
- Murakami, M.; Miyasaka, H.; Okada, T.; Kobatake, S; Irie, M. Dynamics and mechanisms of the multiphoton gated photochromic reaction of diarylethene derivatives. J. Am. Chem. Soc. 2004, 126, 14764–14772. [Google Scholar] [CrossRef]
- Ribeiro Santos, A.; Ballardini, R.; Belser, P.; Gandolfi, M.T.; Iyerd, V.M.; Luca, M. Photochemical investigation of a photochromic diarylethene compound that can be used as a wide range actinometer. Photochem. Photobio. Sci. 2009, 1734–1742. [Google Scholar]
- Maafi, M. The potential of AB(1Φ) systems for direct actinometry. Diarylethenes as successful actinometers for the visible range. Phys. Chem. Chem. Phys. 2010, 12, 13248–13254. [Google Scholar] [CrossRef]
- Pariani, G.; Bianco, A.; Castagna, R.; Bertarelli, C. Kinetics of photochromic conversion at the solid state: Quantum yield of dithienylethene-based film. J. Phys. Chem. A 2011, 115, 12184–12193, and references therein for the QY determination. [Google Scholar] [CrossRef]
- Nakamura, S.; Yokojima, S.; Uchida, K.; Tsujioka, T. Photochromism of diarylethene: Effect of polymer environment and effects on surfaces. J. Photochem. Photobio. C: Photochem. Rev. 2011, 12, 138–150. [Google Scholar] [CrossRef]
- Nakamuram, S.; Yokojima, S.; Uchida, K.; Tsujioka, T.; Goldberg, A.; Murakami, A.; Shinoda, K.; Mikamim, M.; Kobayashi, T.; Kobatake, S.; et al. Theoretical investigation on photochromic diarylethene: A short review. J. Photochem. Photobiol. A: Chem. 2008, 200, 10–18. [Google Scholar] [CrossRef]
- Boggio-Pasqua, M.; Ravaglia, M.; Bearpark, M.J.; Garavelli, M.; Robb, M.A. Can diarylethene photochromism be explained by a reaction path alone? A CASSCF study with Model MMVB dynamics. J. Phys. Chem. A 2003, 107, 11139–11152. [Google Scholar] [CrossRef]
- Tani, K.; Ishibashi, Y.; Miyasaka, M.; Kobatake, S.; Irie, M. Dynamics of cyclization, cycloreversion, and multiphoton-gated reaction of a photochromic diarylethene derivative in crystalline phas. J. Phys. Chem. C 2008, 112, 11150–11157. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Mukaida, M.; Falkenstro, M.; Miyasaka, M.; Kobatake, S.; Irie, M. One- and multi-photon cycloreversion reaction dynamics of diarylethene derivative with asymmetrical structure, as revealed by ultrafast laser spectroscopy. Phys. Chem. Chem. Phys. 2009, 11, 2640–2648. [Google Scholar] [CrossRef]
- Uchida, K.; Guillaumont, D.; Tsuchida, E.; Mochizuki, G.; Irie, M.; Murakami, A.; Nakamura, S. Theoretical study of an intermediate, a factor determining the quantum yield in photochromism of diarylethene derivatives. J. Mol. Struct. 2002, 579, 115–120. [Google Scholar] [CrossRef]
- Asano, Y.; Murakami, A.; Kobayashi, T.; Kobatake, S.; Irie, M.; Yabushita, S.; Nakamura, S. Theoretical study on novel quantum yield of dithienylethenes cyclization reactions in crystals. J. Mol. Struct. 2003, 625, 227–234. [Google Scholar] [CrossRef]
- Tsuboi, Y.; Shimizu, R.; Shoji, T.; Kitamura, N. Near-infrared continuous-wave light driving a two-photon photochromic reaction with the assistance of localized surface plasmon. J. Am. Chem. Soc. 2009, 131, 12623–12627. [Google Scholar]
- Seminario, J. Molecular and Nano Electronics: Analysis, Design and Simulation; Elsevier: Amsterdam, The Netherland, 2006. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nakamura, S.; Uchida, K.; Hatakeyama, M. Potential Energy Surfaces and Quantum Yields for Photochromic Diarylethene Reactions. Molecules 2013, 18, 5091-5103. https://doi.org/10.3390/molecules18055091
Nakamura S, Uchida K, Hatakeyama M. Potential Energy Surfaces and Quantum Yields for Photochromic Diarylethene Reactions. Molecules. 2013; 18(5):5091-5103. https://doi.org/10.3390/molecules18055091
Chicago/Turabian StyleNakamura, Shinichiro, Kingo Uchida, and Makoto Hatakeyama. 2013. "Potential Energy Surfaces and Quantum Yields for Photochromic Diarylethene Reactions" Molecules 18, no. 5: 5091-5103. https://doi.org/10.3390/molecules18055091




