The Effect of PAMAM Dendrimers on the Antibacterial Activity of Antibiotics with Different Water Solubility
Abstract
:1. Introduction
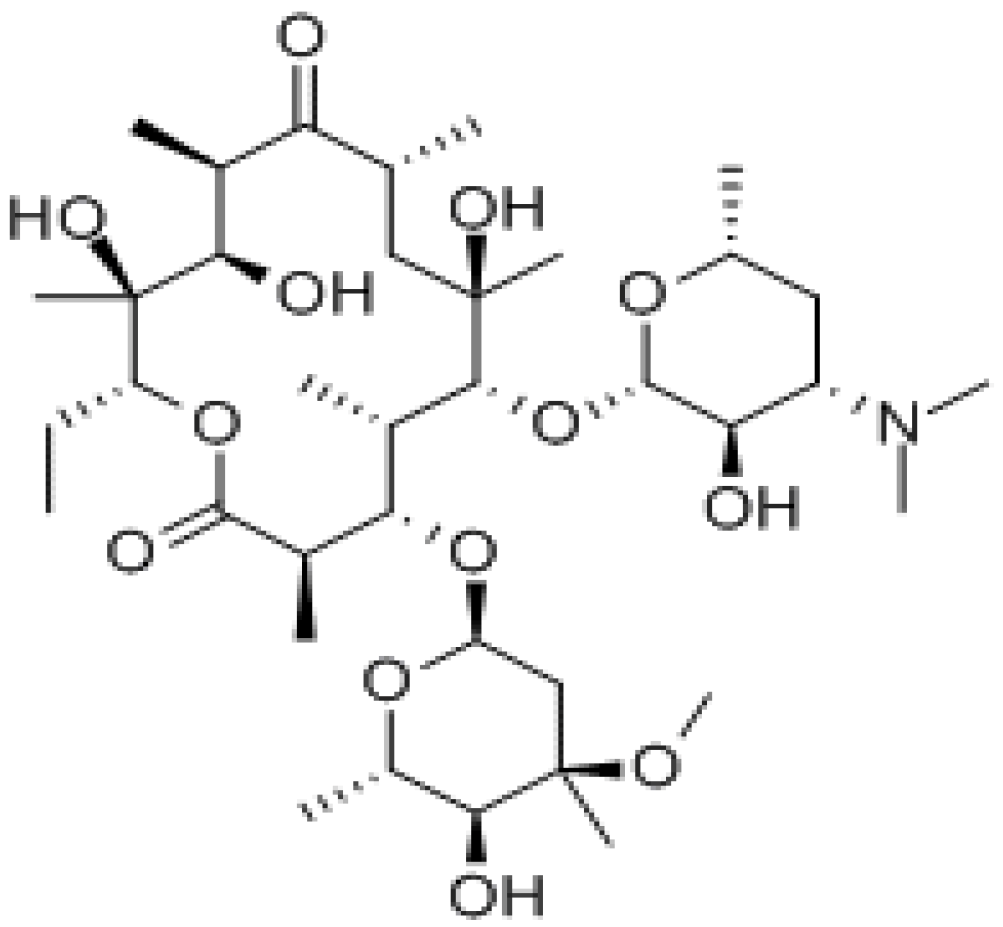
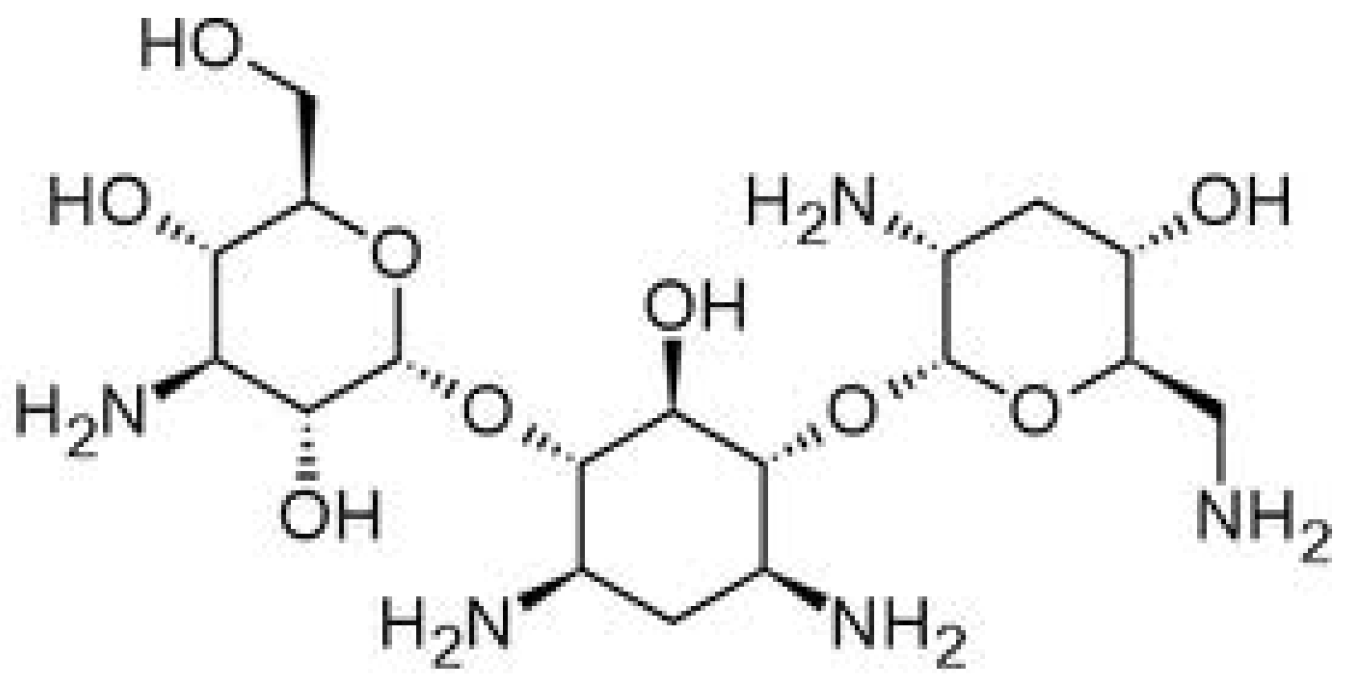
2. Results and Discussion
2.1. Influence of PAMAM Dendrimers on Antibacterial Activity of Erythromycin and Tobramycin
| Name of the strain | MIC (µg/mL) | ||||
|---|---|---|---|---|---|
| EM | EM + PAMAM-NH2 | EM + PAMAM-OH | |||
| G2 | G3 | G2 | G3 | ||
| Staphylococcus aureus ATCC 29213 G(+) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Enterococcus faecalis ATCC 29212 G(+) | 1 | 1 | 1 | 1 | 1 |
| Staphylococcus aureus (clinical strain) G(+) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Enterococcus faecalis (clinical strain) G(+) | >512 | >512 | >512 | >512 | >512 |
| Escherichia coli ATCC 25922* G(−) | − | − | − | − | − |
| Pseudomonas aeruginosa ATCC 27853* G(−) | − | − | − | − | − |
| Acinetobacter baumannii LMG 1025* G(−) | − | − | − | − | − |
| Klebsiella pneumonia ATCC 700603* G(−) | − | − | − | − | − |
| Enterobacter cloacae ATCC 700323* G(−) | − | − | − | − | − |
| Name of the strain | MBC (µg/mL) | |||||
|---|---|---|---|---|---|---|
| EM | EM + PAMAM-NH2 | EM + PAMAM-OH | ||||
| G2 | G3 | G2 | G3 | |||
| Staphylococcus aureus ATCC 29213 G(+) | 16 | 8 | 8 | 16 | 8 | |
| Enterococcus faecalis ATCC 29212 G(+) | 8 | 8 | 8 | 8 | 8 | |
| Staphylococcus aureus (clinical strain) G(+) | 16 | 4 | 4 | 16 | 8 | |
| Enterococcus faecalis (clinical strain) G(+) | >512 | >512 | >512 | >512 | >512 | |
| Escherichia coli ATCC 25922* G(−) | − | − | − | − | − | − |
| Pseudomonas aeruginosa ATCC 27853* G(−) | − | − | − | − | − | − |
| Acinetobacter baumannii LMG 1025* G(−) | − | − | − | − | − | − |
| Klebsiella pneumonia ATCC 700603* G(−) | − | − | − | − | − | − |
| Enterobacter cloacae ATCC 700323* G(−) | − | − | − | − | − | − |
| Name of the strain | MIC (µg/mL) | ||||
|---|---|---|---|---|---|
| TOB | TOB + PAMAM-NH2 | TOB + PAMAM-OH | |||
| G2 | G3 | G2 | G3 | ||
| Staphylococcus aureus ATCC 29213 G(+) | 1 | 1 | 1 | 1 | 2 |
| Enterococcus faecalis ATCC 29212 G(+) | 16 | 32 | 32 | 16 | 16 |
| Staphylococcus aureus (clinical strain) G(+) | 1 | 1 | 1 | 1 | 1 |
| Enterococcus faecalis (clinical strain) G(+) | >512 | >512 | >512 | >512 | >512 |
| Escherichia coli ATCC 25922 G(−) | 1 | 1 | 1 | 2 | 2 |
| Pseudomonas aeruginosa ATCC 27853 G(−) | 0.25 | 0.5 | 0.25 | 0.25 | 0.25 |
| Acinetobacter baumannii LMG 1025 G(−) | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 |
| Klebsiella pneumonia ATCC 700603 G(−) | 4 | 4 | 4 | 4 | 4 |
| Enterobacter cloacae ATCC 700323 G(−) | 2 | 2 | 2 | 2 | 2 |
| Name of the strain | MBC (µg/mL) | ||||
|---|---|---|---|---|---|
| TOB | TOB + PAMAM-NH2 | TOB + PAMAM-OH | |||
| G2 | G3 | G2 | G3 | ||
| Staphylococcus aureus ATCC 29213 G(+) | 4 | 4 | 4 | 4 | 4 |
| Enterococcus faecalis ATCC 29212 G(+) | 16 | 64 | 64 | 16 | 16 |
| Staphylococcus aureus (clinical strain) G(+) | 4 | 4 | 4 | 4 | 4 |
| Enterococcus faecalis (clinical strain) G(+) | >512 | >512 | >512 | >512 | >512 |
| Escherichia coli ATCC 25922 G(−) | 1 | 1 | 1 | 2 | 2 |
| Pseudomonas aeruginosa ATCC 27853 G(−) | 0.25 | 0.5 | 0.25 | 0.25 | 0.25 |
| Acinetobacter baumannii LMG 1025 G(−) | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Klebsiella pneumonia ATCC 700603 G(−) | 4 | 4 | 4 | 8 | 4 |
| Enterobacter cloacae ATCC 700323 G(−) | 2 | 2 | 2 | 2 | 2 |
2.2. The Influence of PAMAM Dendrimers on Solubility of Erythromycin

3. Experimental
3.1. Materials
3.2. Bacterial Strains
3.3. Antibacterial Agents
3.4. Determination of Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
3.5. Solubility Studies of Erythromycin
3.6. HPLC Analysis of Erythromycin
3.7. Data Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- European Pharmacopoeia, 6th ed.; Council of Europe: Strasbourg, France, 2007; Volume 2, pp. 1801–1803.
- Mazzei, T.; Mini, E.; Novelli, A.A. Chemistry and mode of action of macrolides. J. Antimicrob. Chemother. 1993, 31, 1–9. [Google Scholar] [CrossRef]
- European Pharmacopoeia, 6th ed.; Council of Europe: Strasbourg, France, 2007; Volume 2, pp. 3085–3086.
- Mingeot-Leclerq, M-P.; Glupczynski, Y.; Tulkens, P.M. Aminoglycosides: activity and resistance. Antimicrob. Agents Chemother. 1999, 43, 727–737. [Google Scholar]
- Chambers, H.F.; Sande, M.A. Antimicrobial agents: The aminoglycosides. In The Pharmacological Basis of Therapeutics; Hardman, J.G., Limbrid, L.E., Molinoff, P.B., Ruddon, R.W., Goodman-Gilman, A., McGraw-Hill, Eds.; McGraw-Hill Professional: New York, NY, 1995; pp. 1103–1121. [Google Scholar]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2012, 64, 102–115. [Google Scholar] [CrossRef]
- Caminade, A.M.; Laurent, R.; Majoral, J.P. Characterization of dendrimers. Adv. Drug Deliv. Rev. 2005, 57, 2130–2146. [Google Scholar] [CrossRef]
- Gillies, E.R.; Frechet, J.M. Dendrimers and dendritic polymers in drug delivery. Drug Discov. Today 2005, 10, 35–43. [Google Scholar] [CrossRef]
- Florence, A.T. Dendrimers: A versatile targeting platform. Adv. Drug Deliv. Rev. 2005, 57, 2104–2105. [Google Scholar] [CrossRef]
- Polcyn, P.; Jurczak, M.; Rajnisz, A.; Solecka, J.; Urbańczyk-Lipkowska, Z. Design of antimicrobially active small amphiphilic peptide dendrimers. Molecules 2009, 14, 3881–3905. [Google Scholar] [CrossRef]
- Janiszewska, J.; Sowińska, M.; Rajnisz, A.; Solecka, J.; Łącka, I.; Milewski, S.; Urbańczyk-Lipkowska, Z. Novel dendrimeric lipopeptides with antifungal activity. Bioorg. Med. Chem. Lett. 2012, 22, 1388–1393. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef]
- Winnicka, K.; Sosnowska, K.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E. Poly(amidoamine) dendrimers increase antifungal activity of clotrimazole. Biol. Pharm. Bull. 2011, 34, 1129–1133. [Google Scholar] [CrossRef]
- Gardiner, J.; Freeman, S.; Leah, M.; Green, A.; Alcock, J.; D’Emanuele, A. PAMAM dendrimers for the delivery of the antibacterial Triclosan. J. Enzyme Inhib. Med. Chem. 2008, 23, 623–628. [Google Scholar] [CrossRef]
- Roseita, E.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Winnicka, K.; Bielawski, K.; Rusak, M.; Bielawska, A. The effect of generation 2 and 3 poly(amidoamine) dendrimers on viability of human breast cancer cells. J. Health. Sci. 2009, 55, 169–177. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, Z.; Ma, M.; Xu, T. Dendrimers as drug carriers: Applications in different routes of drug administration. J. Pharm. Sci. 2008, 97, 123–143. [Google Scholar] [CrossRef]
- Cheng, Y.; Qu, H.; Ma, M.; Xu, Z.; Xu, P.; Fang, Y.; Xu, T.; Wen, L. Polyamidoamine (PAMAM) dendrimers as biocompatible carriers of quinolone antimicrobials: An in vitro study. Europ. J. Med. Chem. 2007, 42, 1032–1038. [Google Scholar] [CrossRef]
- Ma, M.; Cheng, Y.; Xu, Z.; Xu, P.; Qu, H.; Fang, Y.; Xu, T.; Wen, L. Evaluation of polyamidoamine (PAMAM) dendrimers as drug carriers of anti-bacterial drugs using sulfamethoxazole (SMZ) as model drug. Eur. J. Med. Chem. 2007, 42, 93–98. [Google Scholar] [CrossRef]
- Charles, S.; Vasanthan, N.; Kwon, D.; Sekosan, G.; Ghosh, S. Surface modification of poly(amidoamine) (PAMAM) dendrimer as antimicrobial agents. Tetrahedron Lett. 2012, 53, 6670–6675. [Google Scholar] [CrossRef]
- Strydom, S.J.; Rose, W.E.; Otto, D.P.; Liebenberg, W.; de Villiers, M.M. Poly(amidoamine) dendrimer-mediated synthesis and stabilization of silver sulfonamide nanoparticles with increased antibacterial activity. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 85–93. [Google Scholar] [CrossRef]
- McNerny, D.Q.; Leroueil, P.R.; Baker, J.R. Understanding specific and nonspecific toxicities: A requirement for the development of dendrimer-based pharmaceuticals. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 249–259. [Google Scholar] [CrossRef]
- Lopez, A.I.; Reins, R.Y.; McDermott, A.M.; Trautner, B.W.; Cai, C. Antibacterial activity and cytotoxicity of PEGylated poly(amidoamine) dendrimers. Mol. Biosyst. 2009, 5, 1148–1156. [Google Scholar] [CrossRef]
- Ortega, P.; Copa-Patino, J.L.; Munoz-Fernandez, M.A.; Soliveri, J.; Gomez, R.; Mata, F.J. Amine and ammonium functionalization of chloromethylsilane-ended dendrimers. Antimicrobial activity studies. Org. Biomol. Chem. 2008, 6, 3264–3269. [Google Scholar] [CrossRef]
- Balogh, L.; Swanson, D.R.; Tomalia, D.A.; Hagnauer, G.L.; McManus, A.T. Dendrimer-Silver complexes and nanocomposites as antimicrobial agents. Nano. Lett. 2001, 1, 18–21. [Google Scholar] [CrossRef]
- Winnicka, K.; Wroblewska, M.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E. Hydrogel of ketoconazole and PAMAM dendrimers: formulation and antifungal activity. Molecules 2012, 17, 4612–4624. [Google Scholar] [CrossRef]
- Hong, S.; Bielinska, A.U.; Mecke, A.; Keszler, B.; Beals, J.; Shi, X.; Balogh, L.; Orr, B.G.; Baker, J.R., Jr.; Banaszak Holl, M.M. Interactions of poly(amidoamine) dendrimers with supported lipid bilayer and cells: hole formation and the relation to transport. Bioconjugat. Chem. 2004, 15, 774–782. [Google Scholar] [CrossRef]
- Chen, C.Z.; Cooper, S.L. Interactions between dendrimer biocides and bacterial membranes. Biomaterials 2002, 23, 3359–3368. [Google Scholar] [CrossRef]
- Sayed, M.E.; Ginski, M.; Rhodes, H. Transepithelial transport of polyamidoamine dendrimers across Caco-2 cell monolayers. J. Control. Release 2002, 81, 355–365. [Google Scholar] [CrossRef]
- Xue, X.; Chen, X.; Mao, X.; Hou, Z.; Zhou, Y.; Bai, H.; Meng, J.; Da, F.; Sang, G.; Wang, Y.; Luo, X. Amino-terminated generation 2 poly(amidoamine) dendrimer as a potential broad-spectrum, nonresistance-inducing antibacterial agent. AAPS J. 2013, 15, 132–142. [Google Scholar] [CrossRef]
- Calabretta, M.K.; Kumar, A.; McDermott, A.M.; Cai, C. Antibacterial activities of poly(amidoamine) dendrimers terminated with amino and poly(ethylene glycol) groups. Biomacromolecules 2007, 8, 1807–1811. [Google Scholar] [CrossRef]
- Wang, B.; Navath, R.S.; Menjoge, A.R.; Balakrishnan, B.; Bellair, R.; Dai, H.; Romero, R.; Kannan, S.; Kannan, R.M. Inhibition of bacterial growth and intramniotic infection in a guinea pig model of chorioamnionitis using PAMAM dendrimers. Int. J. Pharm. 2010, 395, 298–308. [Google Scholar] [CrossRef]
- Scott, J.R.; Barnett, T.C. Surface proteins of gram-positive bacteria and how they get there. Annu. Rev. Microbiol. 2006, 60, 397–423. [Google Scholar] [CrossRef]
- Cabeen, M.T.; Jacobs-Wagner, C. Bacterial cell shape. Nat. Rev. Microbiol. 2005, 3, 601–610. [Google Scholar] [CrossRef]
- Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. In Processing of European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID); EUCAST Discussion Document E.Dis 5.1. 2003.
- Kamarei, F.; Movaghari, F.; Ghaffari, A.; Bozchalooi, I.S.; Zamani, A.; Jabbari, A. Development of stability-indicating high performance liquid chromathography method for assay of erythromycin ethylosuccinate in powder for oral suspension dosage form. Arab. J. Chem. 2011, 30, 1–7. [Google Scholar]
- Kanfer, I.; Skinner, M.F.; Walker, R.B. Analysis of macrolide antibiotics. J. Chromatogr. A 1998, 812, 255–286. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Winnicka, K.; Wroblewska, M.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E.A. The Effect of PAMAM Dendrimers on the Antibacterial Activity of Antibiotics with Different Water Solubility. Molecules 2013, 18, 8607-8617. https://doi.org/10.3390/molecules18078607
Winnicka K, Wroblewska M, Wieczorek P, Sacha PT, Tryniszewska EA. The Effect of PAMAM Dendrimers on the Antibacterial Activity of Antibiotics with Different Water Solubility. Molecules. 2013; 18(7):8607-8617. https://doi.org/10.3390/molecules18078607
Chicago/Turabian StyleWinnicka, Katarzyna, Magdalena Wroblewska, Piotr Wieczorek, Pawel Tomasz Sacha, and Elzbieta Anna Tryniszewska. 2013. "The Effect of PAMAM Dendrimers on the Antibacterial Activity of Antibiotics with Different Water Solubility" Molecules 18, no. 7: 8607-8617. https://doi.org/10.3390/molecules18078607





