An Azabisphosphonate-Capped Poly(phosphorhydrazone) Dendrimer for the Treatment of Endotoxin-Induced Uveitis
Abstract
:1. Introduction
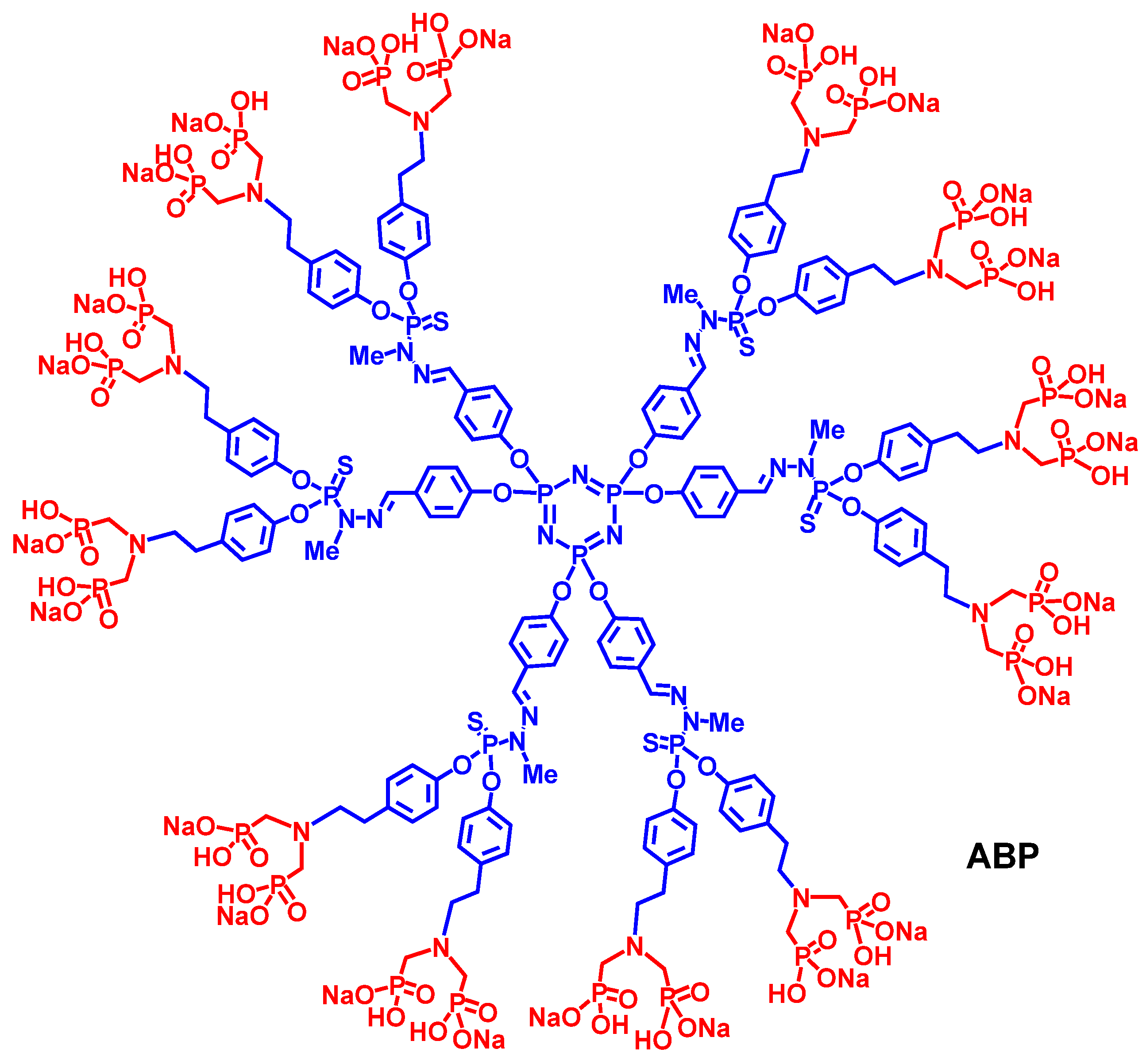
2. Results and Discussion
2.1. Ocular Tolerability of Dendrimer ABP
| Group/Animal number | score | Group/Animal number | score | |
|---|---|---|---|---|
| Group 1/102 (RE) | 0 | Group 3/301 (RE) | 3 | |
| Group 1/102 (LE) | 0 | Group 3/301 (LE) | 1 | |
| Group 1/103 (RE) | 0 | Group 3/302 (RE) | 2 | |
| Group 1/103 (LE) | 0 | Group 3/302 (LE) | 3 | |
| Group 2/201 (RE) | 0 | Group 3/303 (RE) | 2 | |
| Group 2/201 (LE) | 0 | Group 3/303 (LE) | 2 | |
| Group 2/203 (RE) | 0 | Group 3/304 (RE) | 2 | |
| Group 2/203 (LE) | 0 | Group 3/305 (LE) | 1 |
2.2. Efficacy of Dendrimer ABP to Treat Endotoxin-Induced Uveitis in Rats
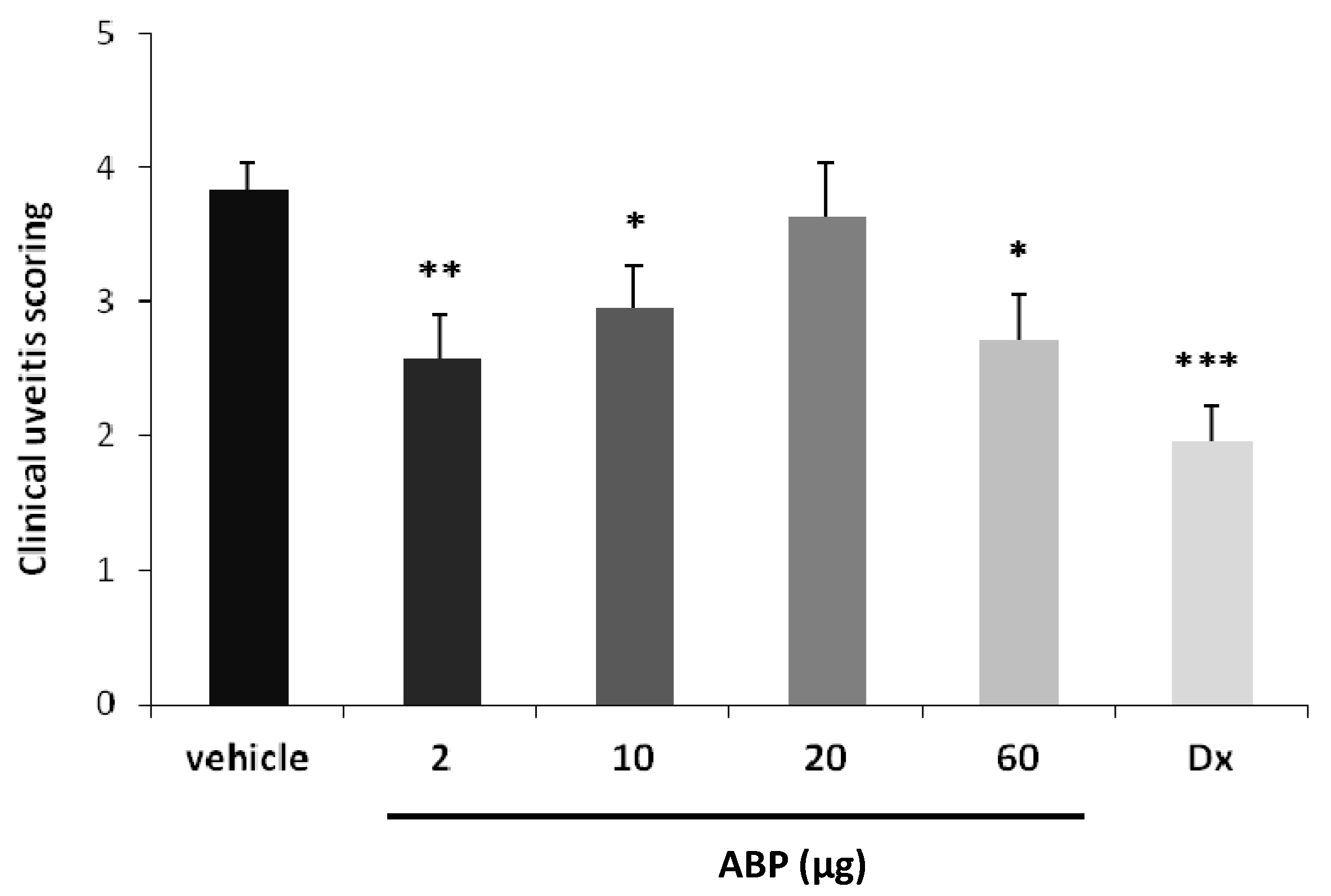
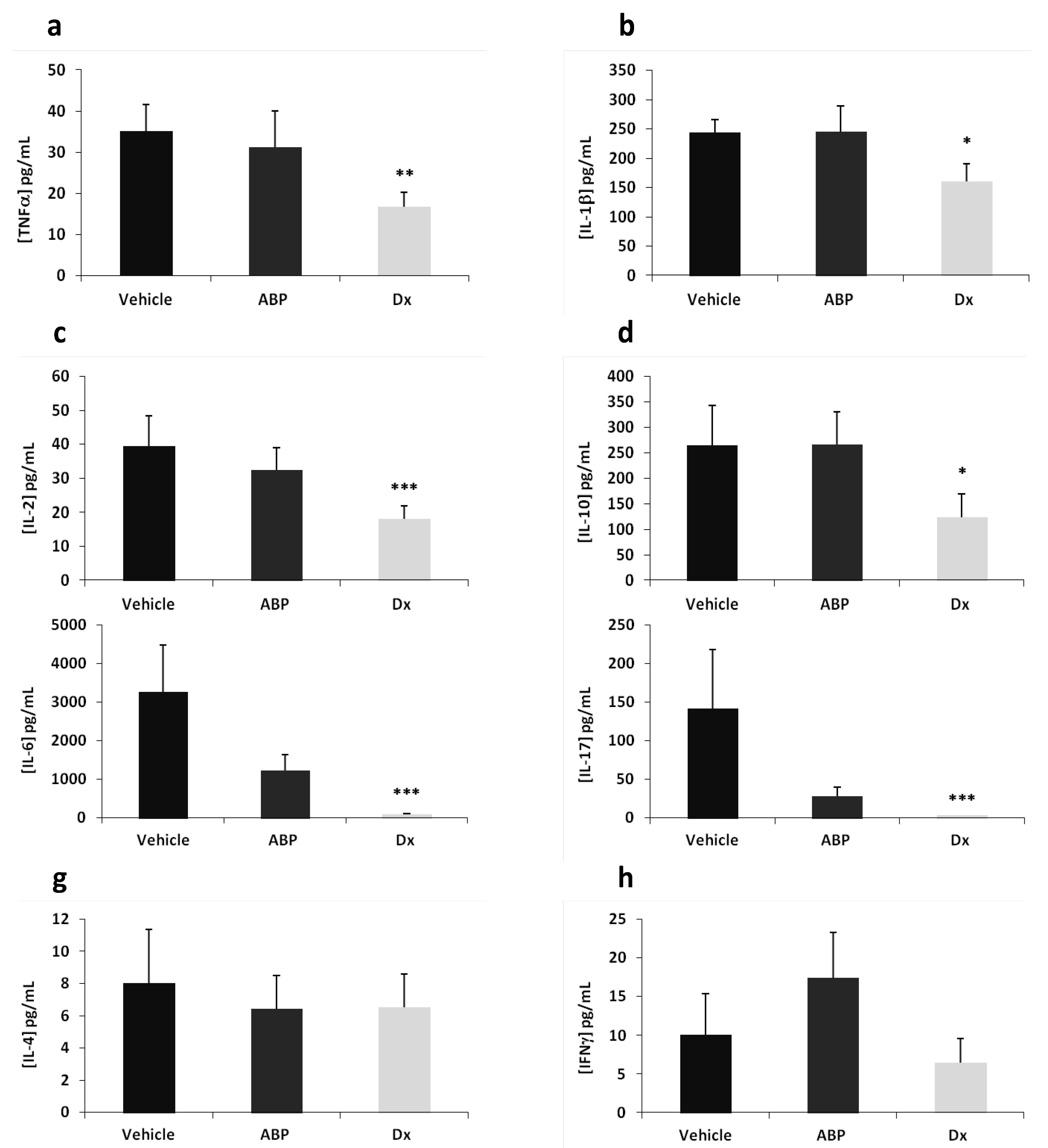
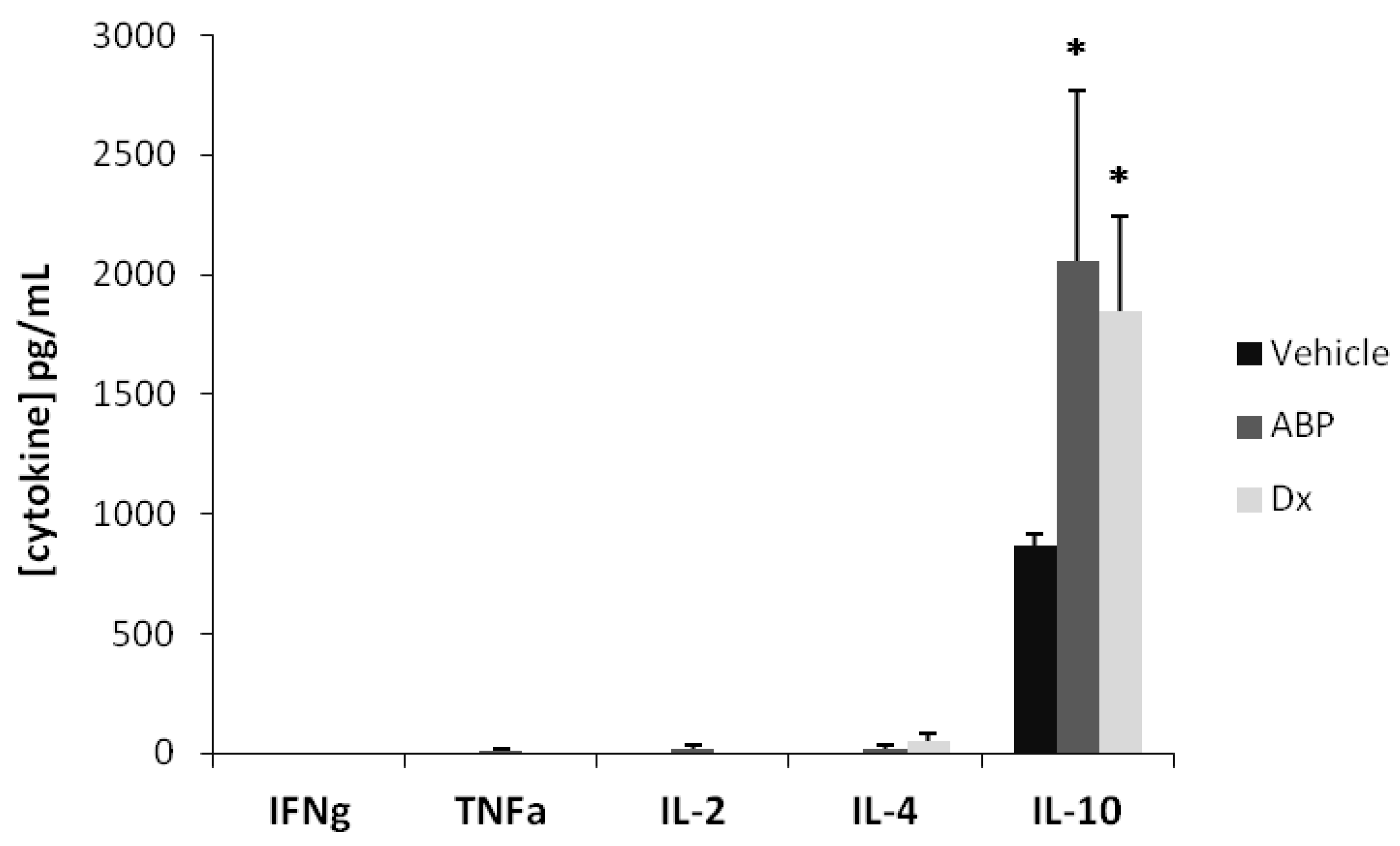
3. Experimental
3.1. Synthesis of Dendrimer ABP
3.2. Animals
3.3. Ocular Tolerability Study
3.4. “Proof of Concept” in an EIU Rat Model
3.5. Measurement of Cytokine Concentrations in Ocular Fluids
3.6. Measurement of Cytokine Concentrations in Serum
3.7. Statistical Analyses
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Caminade, A.M.; Laurent, R.; Zablocka, M.; Majoral, J.P. Organophosphorus chemistry for the synthesis of dendrimers. Molecules 2012, 17, 13605–13621. [Google Scholar] [CrossRef]
- Caminade, A.M.; Turrin, C.O.; Majoral, J.P. Biological properties of phosphorus dendrimers. New J. Chem. 2010, 34, 1512–1524. [Google Scholar] [CrossRef]
- Griffe, L.; Poupot, M.; Marchand, P.; Maraval, A.; Turrin, C.O.; Rolland, O.; Métivier, P.; Bacquet, G.; Fournié, J.J.; Caminade, A.M.; et al. Multiplication of human Natural Killer cells by nanosized phosphonate-capped dendrimers. Angew. Chem. Int. Ed. 2007, 46, 2523–2526. [Google Scholar] [CrossRef]
- Poupot, M.; Griffe, L.; Marchand, P.; Maraval, A.; Rolland, O.; Martinet, L.; L’Faqihi-Olive, F.E.; Turrin, C.O.; Caminade, A.M.; Fournié, J.J.; et al. Design of phosphorylated dendritic architectures to promote human monocyte activation. FASEB J. 2006, 20, 2339–2351. [Google Scholar] [CrossRef]
- Fruchon, S.; Poupot, M.; Martinet, L.; Turrin, C.O.; Majoral, J.P.; Fournié, J.J.; Caminade, A.M.; Poupot, R. Anti-Inflammatory and immuno-suppressive activation of human monocytes by a bio-active dendrimer. J. Leukoc. Biol. 2009, 85, 553–562. [Google Scholar]
- Rolland, O.; Griffe, L.; Poupot, M.; Maraval, A.; Ouali, A.; Coppel, Y.; Fournié, J.J.; Bacquet, G.; Turrin, C.O.; Caminade, A.M.; et al. Tailored control and optimization of the number of phosphonic acid termini on phosphorus-containing dendrimers for the ex vivo activation of human monocytes. Chem. Eur. J. 2008, 14, 4836–4850. [Google Scholar] [CrossRef]
- Marchand, P.; Griffe, L.; Poupot, M.; Turrin, C.O.; Bacquet, G.; Fournié, J.J.; Majoral, J.P.; Poupot, R.; Caminade, A.M. Dendrimers ended by non-symmetrical azadiphosphonate groups: synthesis and immunological properties. Bioorg. Med. Chem. Lett. 2009, 19, 3963–3966. [Google Scholar] [CrossRef]
- Rolland, O.; Turrin, C.O.; Bacquet, G.; Poupot, R.; Poupot, M.; Caminade, A.M.; Majoral, J.P. Efficient synthesis of phosphorus-containing dendrimers capped with isosteric functions of amino-bis(methylene) phosphonic acids. Tetrahedron Lett. 2009, 50, 2078–2082. [Google Scholar] [CrossRef]
- Hayder, M.; Fruchon, S.; Fournié, J.J.; Poupot, M.; Poupot, R. Anti-inflammatory properties of dendrimers per se. Sci. World J. 2011, 11, 1367–1382. [Google Scholar] [CrossRef]
- Hayder, M.; Poupot, M.; Baron, M.; Nigon, D.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P.; Eisenberg, R.A.; Fournié, J.J.; Cantagrel, A.; et al. A phosphorus-based dendrimer targets inflammation and osteoclastogenesis in experimental arthritis. Sci. Transl. Med. 2011, 3, 81ra35. [Google Scholar] [CrossRef]
- Hayder, M.; Poupot, M.; Baron, M.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P.; Eisenberg, R.A.; Fournié, J.J.; Cantagrel, A.; Poupot, R.; et al. Frequency and route of administration in the treatment of experimental arthritis by phosphorus-dendrimer. Ann. Rheum. Dis. 2012. [Google Scholar] [CrossRef]
- Bosch, X. Dendrimers to treat rheumatoid Arthritis. ACS Nano 2011, 5, 6779–6785. [Google Scholar] [CrossRef]
- Wolf, L.K. Dendrimer treats joint inflammation. Chem. Eng. News 2011, 89, 38. [Google Scholar]
- Lou, K.J. Dendrimer throws a blanket on R.A. SciBX 2011, 4, A8. [Google Scholar]
- Touchard, E.; Omri, S.; Naud, M.C.; Berdugo, M.; Deloche, C.; Abadie, C.; Jonet, L.; Jeanny, J.C.; Crisanti, P.; de Kozak, Y.; et al. A peptide inhibitor of c-jun N-terminal kinase for the treatment of endotoxin-induced uveitis. Invest. Ophthalmol. Vis. Sci. 2010, 51, 4683–4693. [Google Scholar] [CrossRef]
- Berdugo, M.; Larsen, I.V.; Abadie, C.; Deloche, C.; Kowalczuk, L.; Touchard, E.; Dubielzig, R.; Brandt, C.R.; Behar-Cohen, F.; Combette, J.M. Ocular distribution, spectrum of activity, and in vivo viral neutralization of a fully humanized anti-herpex simplex virus IgG Fab fragment following topical application. Antimicrob. Agents Chemother. 2012, 56, 1390–1402. [Google Scholar] [CrossRef]
- Chen, F.T.; Liu, Y.C.; Yang, C.M.; Yang, C.H. Anti-inflammatory effect of the proteasome inhibitor Bartezomib on endotoxin-induced uveitis in rats. Invest. Ophthalmol. Vis. Sci. 2012, 53, 3682–3694. [Google Scholar] [CrossRef]
- Rosenbaum, J.T.; McDevitt, H.O.; Guss, R.B.; Egbert, R. Endotoxin-induced uveitis in rats as a model for human disease. Nature 1980, 268, 611–613. [Google Scholar]
- Kaminskas, L.J.; Porter, C.J.H. Targeting the lymphatics using dendritic polymers (dendrimers). Adv. Drug Del. Rev. 2011, 63, 890–900. [Google Scholar] [CrossRef]
- European convention on animal protection for scientific experimentation of 1986. Offic. J. Eur. Commun. 1999, 42, 31–37.
- Ministère des affaires étrangères. Décret Français No.2001-486. (in French). J. Offic. Rep. Fr. 2001, 133, 9094–9120.
- Gaudreault, J.; Fei, D.; Rusit, J.; Suboc, P.; Shiu, V. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol. Vis. Sci. 2005, 46, 726–733. [Google Scholar] [CrossRef]
- Nomoto, H.; Shiraga, F.; Kuno, N.; Kimura, E.; Fujii, S.; Shinomiya, K.; Nugent, A.K.; Hirooka, K.; Baba, B. Pharmacokinetics of Bevacizumab after Topical, Subconjunctival, and Intravitreal Administration in Rabbits. Invest. Ophthalmol. Vis. Sci. 2009, 50, 4807–4813. [Google Scholar] [CrossRef]
- Portevin, D.; Poupot, M.; Rolland, O.; Turrin, C.O.; Fournié, J.J.; Majoral, J.P.; Caminade, A.M.; Poupot, R. Regulatory activity of azabisphosphonate-capped dendrimers on human CD4+ T cell proliferation enhances ex vivo expansion of NK cells from PBMCs for immunotherapy. J. Transl. Med. 2009, 7, 82. [Google Scholar] [CrossRef]
- Sample Availability: Samples of dendrimer ABP are available under MTA from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fruchon, S.; Caminade, A.-M.; Abadie, C.; Davignon, J.-L.; Combette, J.-M.; Turrin, C.-O.; Poupot, R. An Azabisphosphonate-Capped Poly(phosphorhydrazone) Dendrimer for the Treatment of Endotoxin-Induced Uveitis. Molecules 2013, 18, 9305-9316. https://doi.org/10.3390/molecules18089305
Fruchon S, Caminade A-M, Abadie C, Davignon J-L, Combette J-M, Turrin C-O, Poupot R. An Azabisphosphonate-Capped Poly(phosphorhydrazone) Dendrimer for the Treatment of Endotoxin-Induced Uveitis. Molecules. 2013; 18(8):9305-9316. https://doi.org/10.3390/molecules18089305
Chicago/Turabian StyleFruchon, Séverine, Anne-Marie Caminade, Claire Abadie, Jean-Luc Davignon, Jean-Marc Combette, Cédric-Olivier Turrin, and Rémy Poupot. 2013. "An Azabisphosphonate-Capped Poly(phosphorhydrazone) Dendrimer for the Treatment of Endotoxin-Induced Uveitis" Molecules 18, no. 8: 9305-9316. https://doi.org/10.3390/molecules18089305
APA StyleFruchon, S., Caminade, A.-M., Abadie, C., Davignon, J.-L., Combette, J.-M., Turrin, C.-O., & Poupot, R. (2013). An Azabisphosphonate-Capped Poly(phosphorhydrazone) Dendrimer for the Treatment of Endotoxin-Induced Uveitis. Molecules, 18(8), 9305-9316. https://doi.org/10.3390/molecules18089305







