Oligomerization of 10,16-Dihydroxyhexadecanoic Acid and Methyl 10,16-Dihydroxyhexadecanoate Catalyzed by Lipases
Abstract
:1. Introduction
2. Results and Discussion
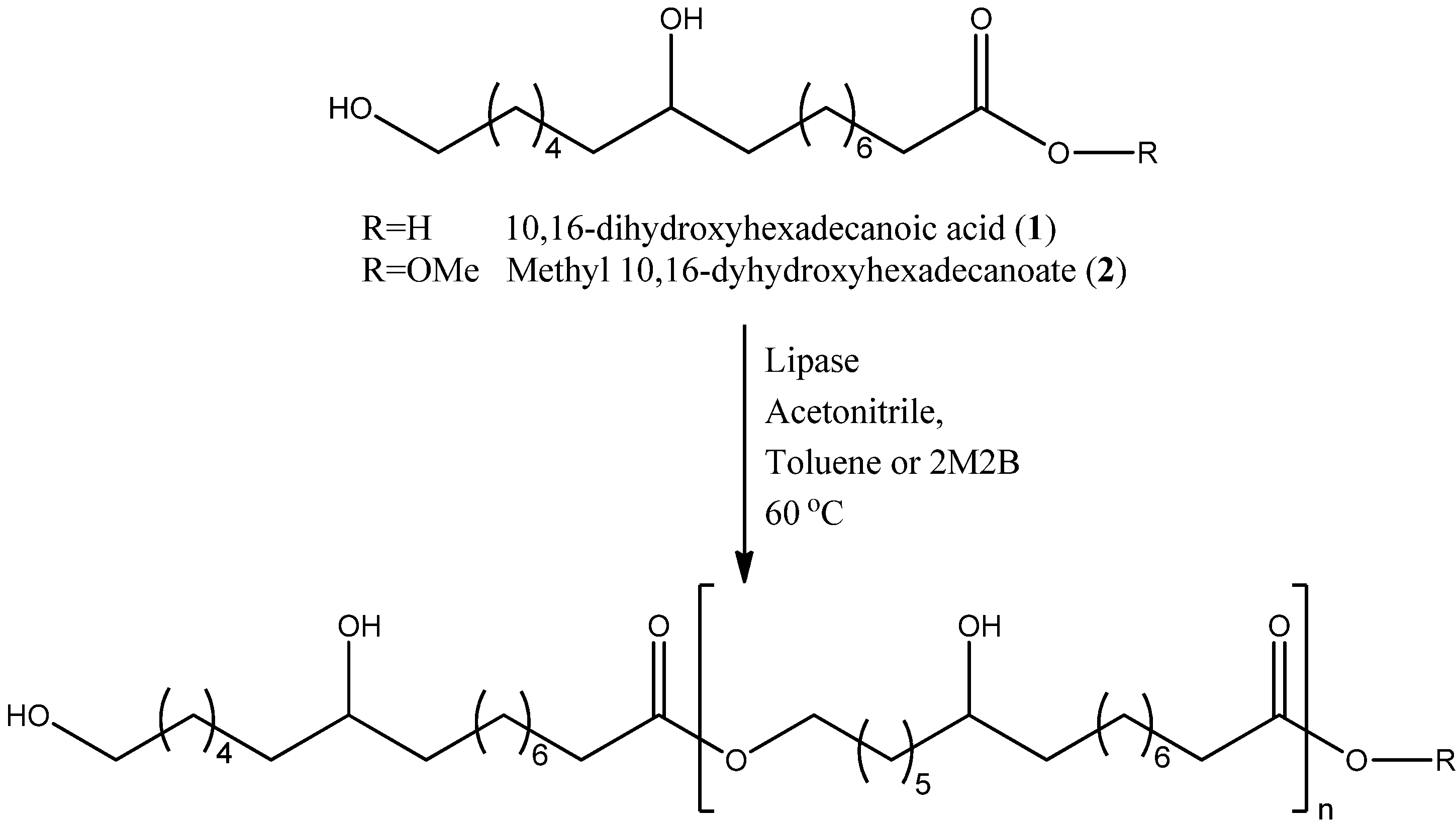
2.1. Enzymatic Polyesterification Studies of the 10,16-DHPA
| Lipase | Toluene | 2M2B |
|---|---|---|
| CAL-B | 80 ± 0.5 | 75 ± 0.9 |
| TL | 65 ± 1.0 | 65 ± 1.2 |
| RM | 64 ± 1.9 | 40 ± 0.7 |
| PPL | 35 ± 1.5 | 25 ± 0.5 |
| PCL | 35 ± 0.9 | 40 ± 1.8 |
| Lipase | Toluene | |
|---|---|---|
| 60 °C | 75 °C | |
| CAL-B | 80 ± 0.9 | 75 ± 0.5 |
| TL | 65 ± 0.8 | 55 ± 1.5 |
| RM | 65 ± 0.7 | 70 ± 1.0 |
| PPL | 35 ± 0.5 | 40 ± 1.7 |
| PCL | 34 ± 1.9 | 40 ± 1.1 |
| Polyester | Monomer | Enzyme | Solvent | Mn | Mw | pd | DP |
|---|---|---|---|---|---|---|---|
| 3 | 1 | Cal-B | Toluene | 814 | 1206 | 1.13 | 2.96 |
| 3 | 1 | RM | Toluene | 771 | 863 | 1.12 | 2.86 |
| 3 | 1 | TL | Toluene | 769 | 857 | 1.13 | 2.97 |
| 4 | 2 | CAL-B | Toluene | 860 | 982 | 1.14 | 3.19 |
| 4 | 2 | CAL-B | Acetonitrile | 653 | 707 | 1.08 | 2.42 |
| 3 | 1 | CAL-B | 2M2B | 565 | 570 | 1.00 | 2.10 |
| 4 | 2 | CAL-B | 2M2B | 605 | 648 | 1.06 | 2.25 |
 ) and methyl-10,16-dihydroxyhexadecanoate (methyl-10,16-DHHD) (
) and methyl-10,16-dihydroxyhexadecanoate (methyl-10,16-DHHD) (  ) in toluene at 60 °C and (b) Extent of chain growth as a function of time of the reaction of 10,16-DHPA (
) in toluene at 60 °C and (b) Extent of chain growth as a function of time of the reaction of 10,16-DHPA (  ) and methyl-10,16-DHHD (
) and methyl-10,16-DHHD (  ). The error bars were calculated from triplicate runs.
). The error bars were calculated from triplicate runs.
 ) and methyl-10,16-dihydroxyhexadecanoate (methyl-10,16-DHHD) (
) and methyl-10,16-dihydroxyhexadecanoate (methyl-10,16-DHHD) (  ) in toluene at 60 °C and (b) Extent of chain growth as a function of time of the reaction of 10,16-DHPA (
) in toluene at 60 °C and (b) Extent of chain growth as a function of time of the reaction of 10,16-DHPA (  ) and methyl-10,16-DHHD (
) and methyl-10,16-DHHD (  ). The error bars were calculated from triplicate runs.
). The error bars were calculated from triplicate runs.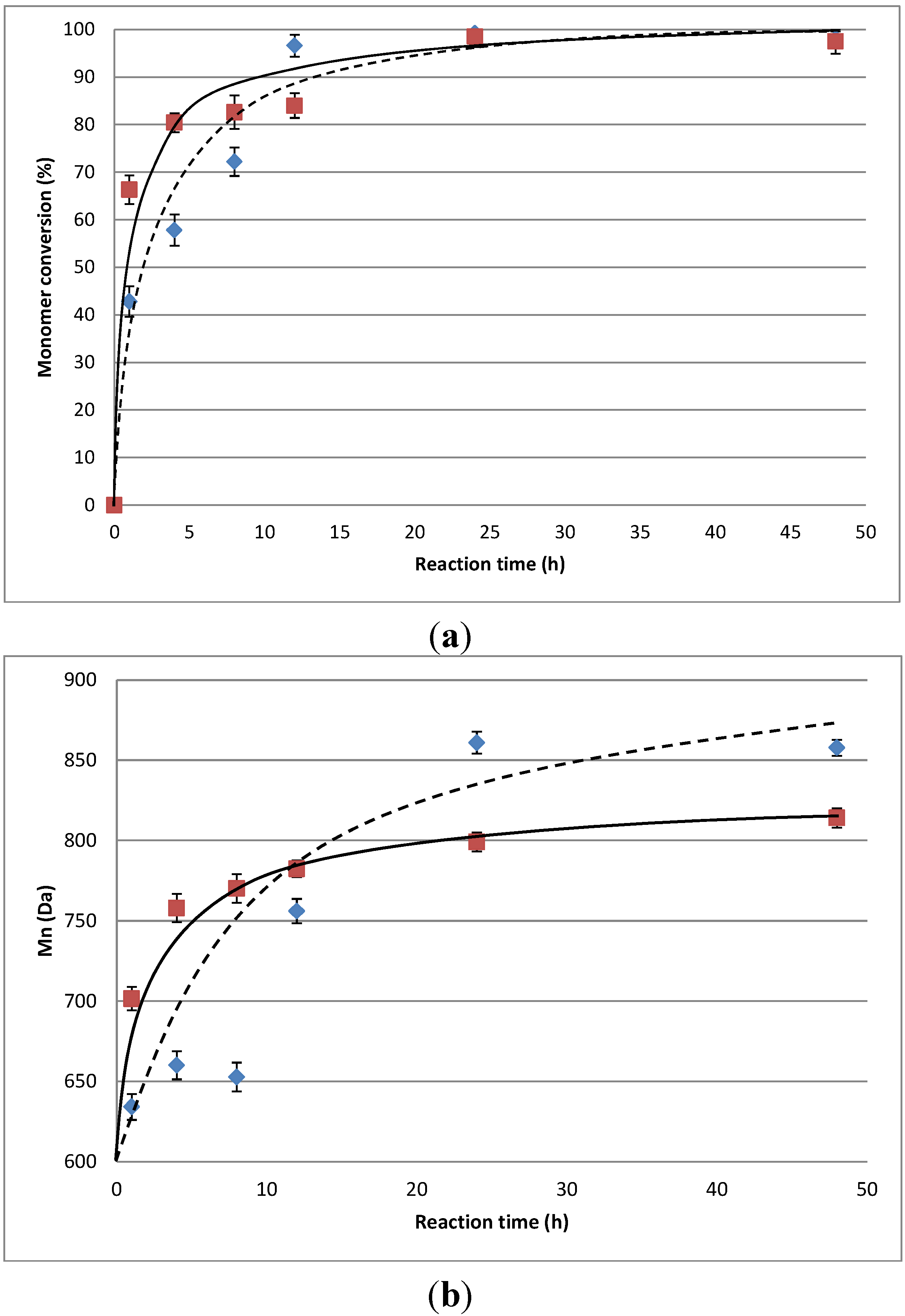
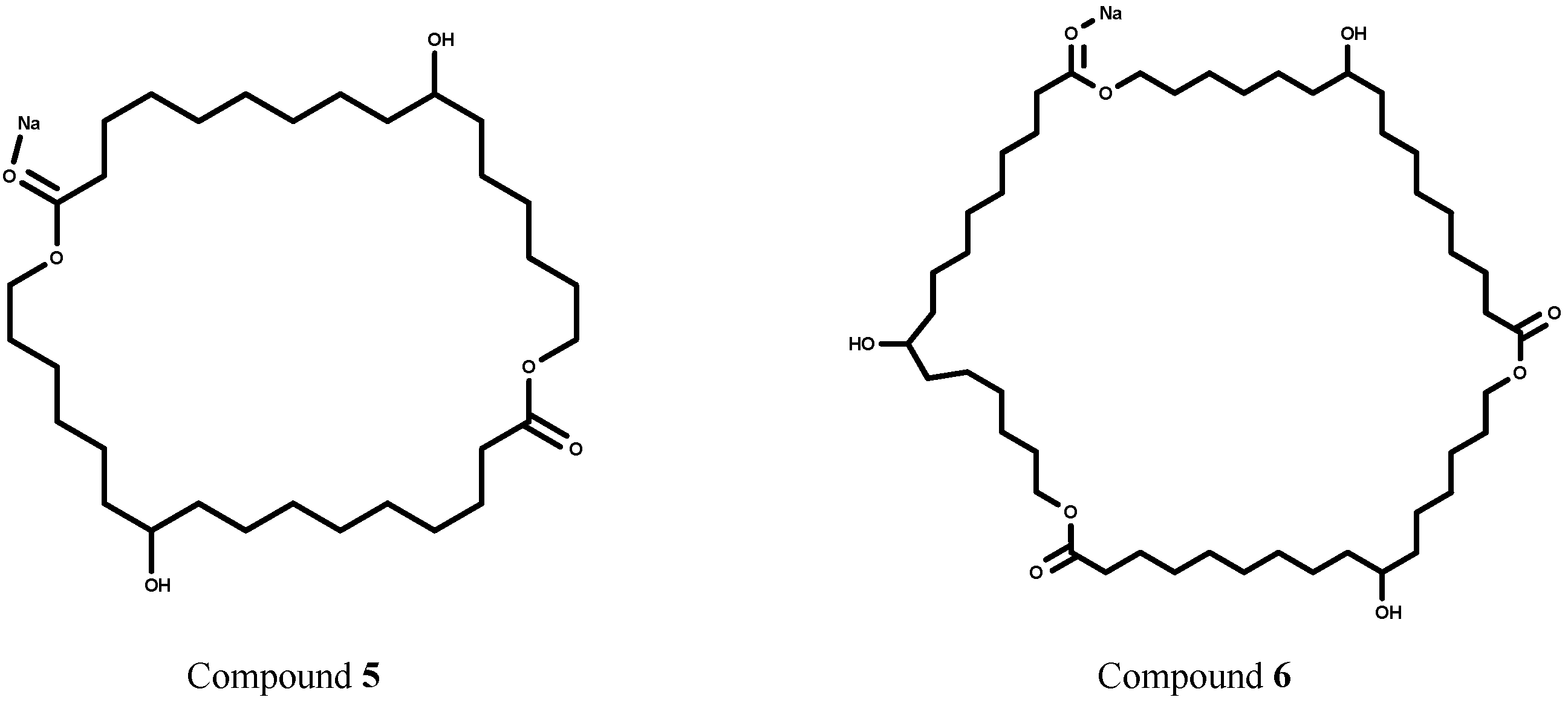
2.2. Enzymatic Trans-Esterification Studies of the Methyl-10,16-Dihydroxyhexadecanoate
2.3. Characterization of the Products
2.3.1. NMR analysis
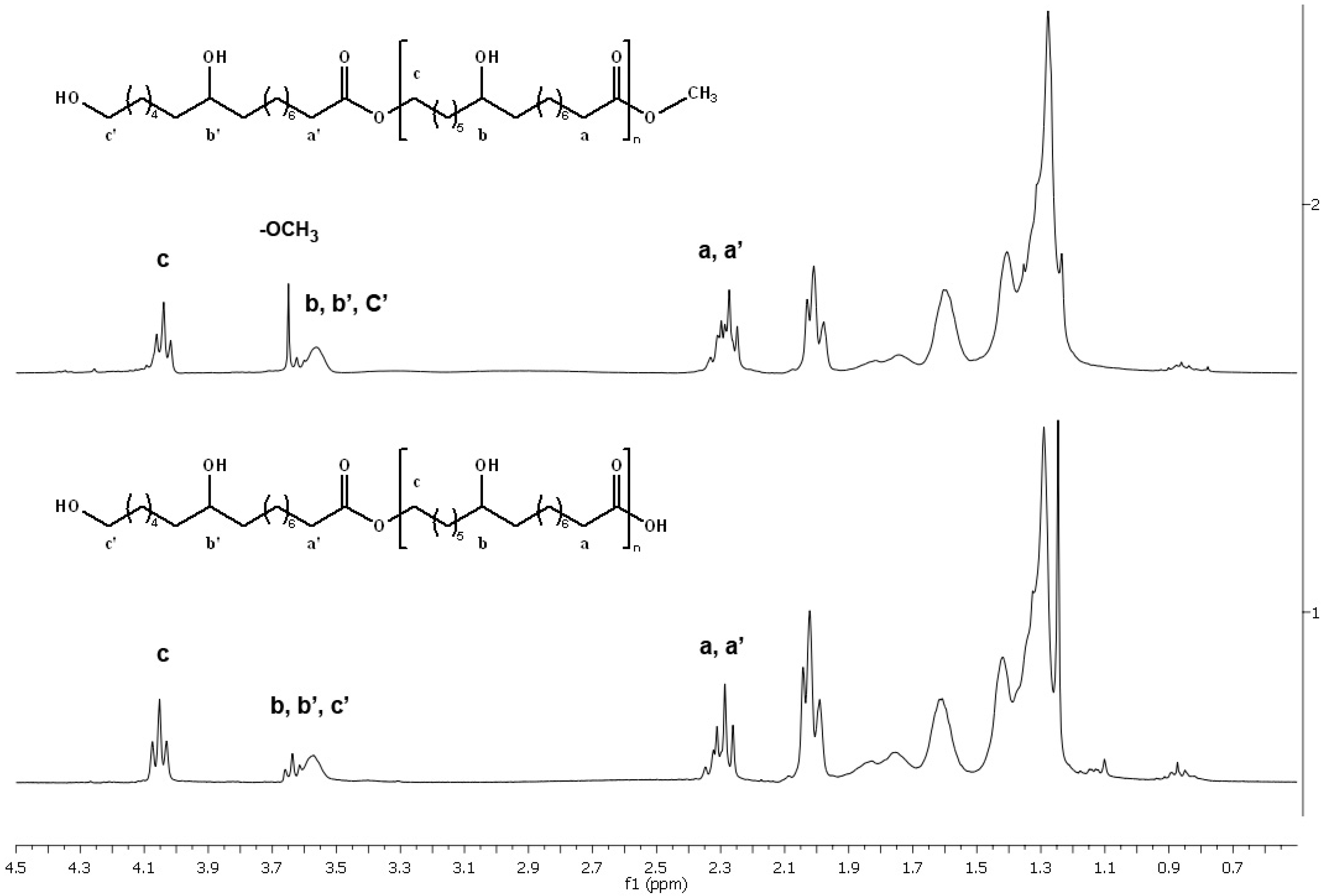
2.3.2. FT-IR Analysis
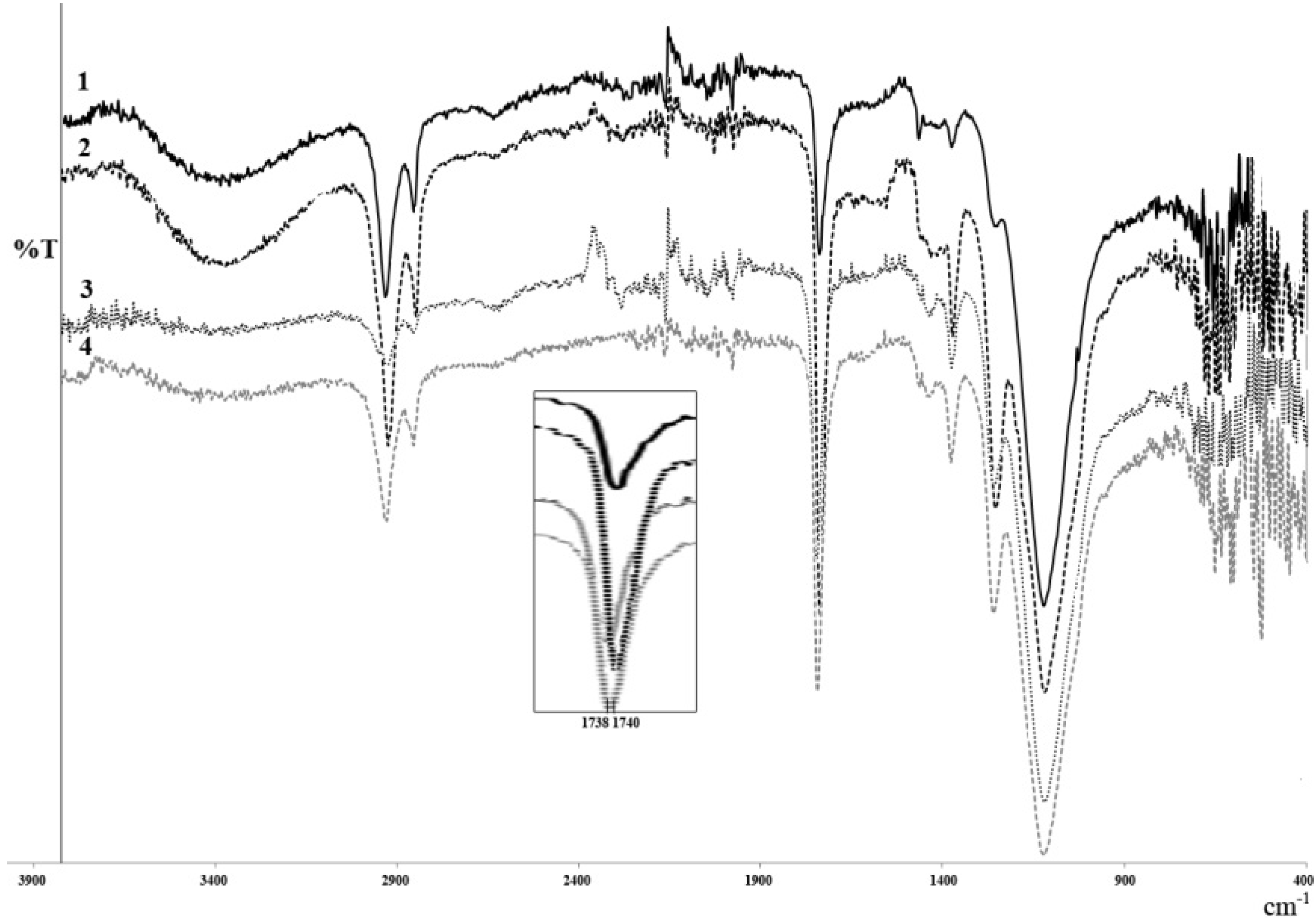
2.3.3. MALDI-TOF MS and ESI MS Analysis

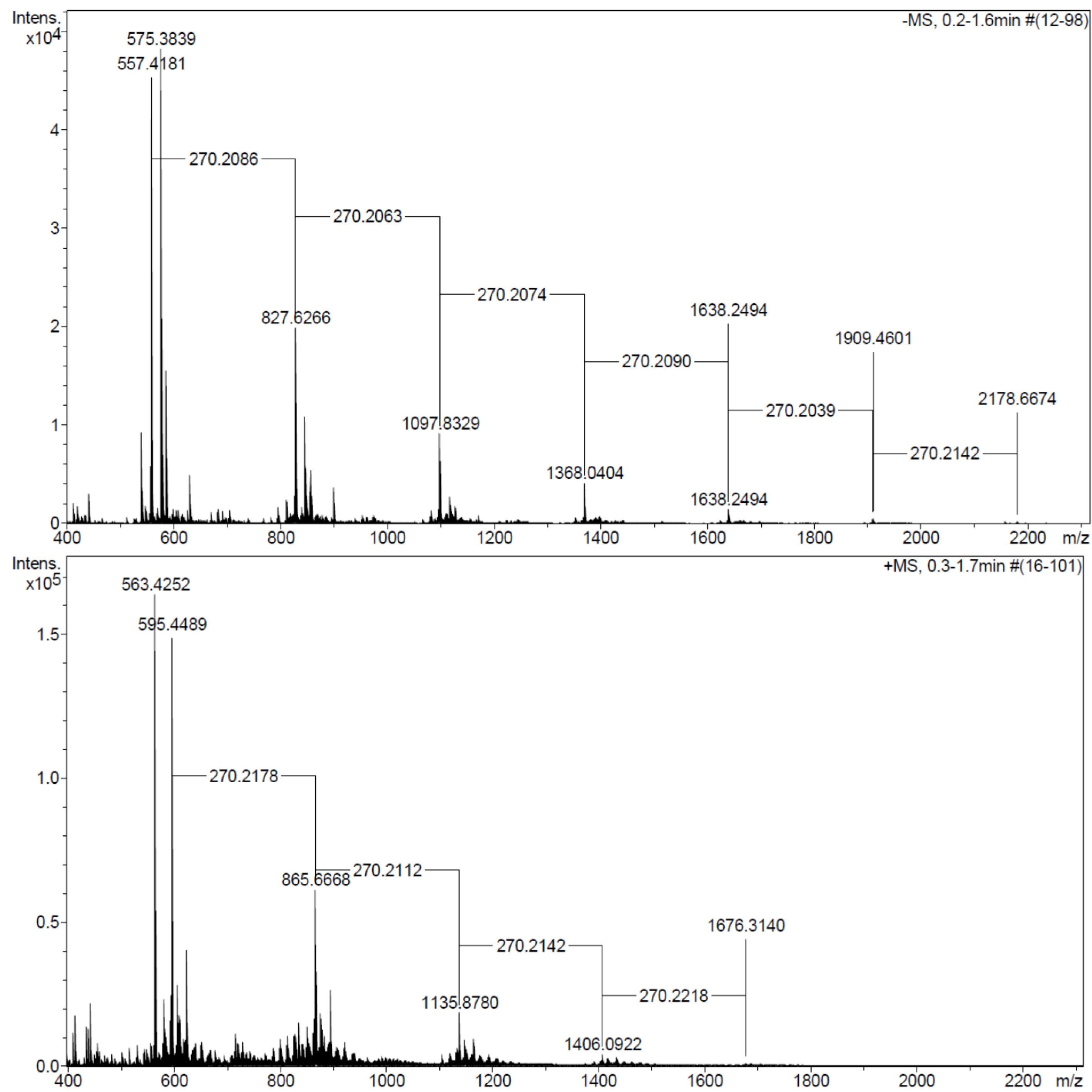
2.3.4. AFM Analysis

3. Experimental
3.1. Materials
3.2. Methods
3.3. Isolation of 10,16-Dihydroxyhexadecanoic Acid (10,16-DHPA, 1)
3.4. Preparation of Methyl 10,16-Dihydroxyhexadecanoate (Methyl-10,16-DHHD, 2)
3.5. Polyesterification of 10,16-DHPA and Trans-Esterification of Methyl-10,16-DHHD
3.6. Conversion of 10,16-DHPA and Methyl-10,16-DHHD by CAL-B
3.7. HPLC Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Song, D.K.; Sung, Y.K. Synthesis and characterization of biodegradable poly(1,4-butanediol succinate). J. Appl. Polym. Sci. 1995, 56, 1381–1395. [Google Scholar] [CrossRef]
- Zhou, S.; Deng, X.; Li, X.; Jia, W.; Liu, L. Synthesis and Characterization of Biodegradable Low Molecular Weight Aliphatic Polyesters and Their Use in Protein-Delivery Systems. J. Appl. Polym. Sci. 2004, 91, 1848–1856. [Google Scholar] [CrossRef]
- Aryal, S.; Prabaharan, M.; Pilla, S.; Gong, S. Biodegradable and biocompatible multi-arm star amphiphilic block copolymer as a carrier for hydrophobic drug delivery. Int. J. Biolog. Macromol. 2009, 44, 346–352. [Google Scholar] [CrossRef]
- Van de Velde, K.; Kiekens, P. Biopolymers: overview of several properties and consequences on their applications. Polym. Testing. 2002, 21, 433–442. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Anderson, J.M. In vivo biocompatibility studies of Medisorb RTM 65/35 D,L-lactide/glycolide copolymer microspheres. J. Control Release 1993, 24, 81–93. [Google Scholar] [CrossRef]
- Langer, R. New methods of drug delivery. Science 1990, 249, 1527–1533. [Google Scholar]
- Wise, D.L.; Fellman, T.D.; Sanderson, J.E.; Wentworth, R.L. Lactide/Glycolide Acid Polymers; Gregoriadis, G., Ed.; Academic Press: London, UK, 1979; p. 237. [Google Scholar]
- Kricheldorf, H.R.; Boettcher, C.; Tonnes, K.U. Polylactones: 23. Polymerization of racemic and meso D,L-lactide with various organotin catalysts—Stereochemical aspects. Polymer 1992, 33, 2817–2824. [Google Scholar] [CrossRef]
- Uhrich, K.E.; Cannizzaro, S.M.; Langer, R.; Shakesheff, K.M. Polymeric systems for controlled drug release. Chem. Rev. 1999, 99, 3181–3198. [Google Scholar] [CrossRef]
- Kline, B.J.; Beckman, E.J.; Russell, A.J. One-step biocatalytic synthesis of linear polyesters with pendant hydroxyl groups. J. Am. Chem. Soc. 1998, 120, 9475–9480. [Google Scholar] [CrossRef]
- Uyama, H.; Kojiro, I.; Kobayashi, S. Regioselectivity Control in Lipase-Catalyzed Polymerization of Divinyl Sebacate and Triols. Macromol. Biosci. 2001, 1, 40–44. [Google Scholar] [CrossRef]
- Kumar, A.; Kulshrestha, A.S.; Gao, W.; Gross, R.A. Versatile Route to Polyol Polyesters by Lipase Catalysis. Macromolecules 2003, 36, 8219–8221. [Google Scholar] [CrossRef]
- Olson, D.A.; Sheares, V.V. Preparation of Unsaturated Linear Aliphatic Polyesters Using Condensation Polymerization. Macromolecules 2006, 39, 2808–2814. [Google Scholar] [CrossRef]
- Carine, R.; de Montigny, F.; Thomas, C.M. Tandem synthesis of alternating polyesters from renewable resources. Nat. Commun. 2011, 2, 586. [Google Scholar] [CrossRef]
- Tschan, M.J.-L.; Brulé, E.; Haquette, P.; Thomas, C.M. Synthesis of biodegradable polymers from renewable resources. Polym. Chem. 2012, 3, 836–851. [Google Scholar] [CrossRef]
- Bernards, M.A.; Lewis, N.G. The macromolecular aromatic domain in suberized tissue: A changing paradigm. Phytochemistry 1998, 47, 915–933. [Google Scholar] [CrossRef]
- Fang, X.; Qiu, F.; Yan, B.; Wang, H.; Mort, A.J.; Stark, R.E. NMR studies of molecular structure in fruit cuticle polyesters. Phytochemistry 2001, 57, 1035–1042. [Google Scholar] [CrossRef]
- Franke, R.; Briesen, I.; Wojciechowski, T.; Faust, A.; Yephremov, A.; Nawrath, C.; Schreiber, L. Apoplastic polyesters in Arabidopsis surface tissues—A typical suberin and a particular cutin. Phytochemistry 2005, 66, 2643–2658. [Google Scholar] [CrossRef]
- Kolattukudy, P.E. Biopolyester membranes of plants: Cutin and suberin. Science 1980, 208, 990–1000. [Google Scholar]
- Osman, S.F.; Irwin, P.; Fett, W.F.; O’Connor, J.V.; Parris, N. Preparation, isolation, and characterization of cutin monomers and oligomers from tomato peels. J. Agric. Food Chem. 1999, 47, 799–802. [Google Scholar] [CrossRef]
- Kolattukudy, P.E. Identification of 16-oxo-9-hydroxy hexadecanoic acid, a novel monomer, as a major component of the biopolymer cutin in embryonic Vicia fab. Biochem. Biophys. Res. Commun. 1972, 49, 1040–1046. [Google Scholar] [CrossRef]
- Peschel, S.; Franke, R.; Schreiber, L.; Knoche, M. Composition of the cuticle of developing sweet cherry fruit. Phytochemistry 2007, 68, 1017–1025. [Google Scholar] [CrossRef]
- Olsson, A.; Lindstrom, M.; Iversen, T. Lipase-Catalyzed Synthesis of an Epoxy-Functionalized Polyester from the Suberin Monomer cis-9,10-Epoxy-18-hydroxyoctadecanoic Acid. Biomacromolecules 2007, 8, 757–760. [Google Scholar] [CrossRef]
- Stark, R.E.; Yan, B.; Ray, A.K.; Chen, Z.; Fang, X.; Garbow, J.R. NMR studies of structure and dynamics in fruit cuticle polyesters. Solid State Nucl. Magn. Reson. 2000, 16, 37–45. [Google Scholar]
- Osman, S.F.; Gerard, H.C.; Fett, W.F.; Moreau, R.A.; Dudley, R.L. Method for the Production and Characterization of Tomato Cutin Oligomers. J. Agric. Food Chem. 1995, 43, 2134–2137. [Google Scholar] [CrossRef]
- Benitez, J.J.; García-Segura, R.; Heredia, A. Plant biopolyester cutin: a tough way to its chemical synthesis. Biochim. Biophys Acta 2004, 1674, 1–3. [Google Scholar]
- Heredia-Guerrero, J.A.; Heredia, A.; García-Segura, R.; Benitez, J.J. Synthesis and characterization of a plant cutin mimetic polymer. Polymer 2009, 50, 5633–5637. [Google Scholar] [CrossRef]
- Kulshrestha, A.S.; Gao, W.; Gross, R.A. Glycerol Copolyesters: Control of Branching and Molecular Weight Using a Lipase Catalyst. Macromolecules 2005, 38, 3193–3204. [Google Scholar] [CrossRef]
- Mahapatro, A.; Kumar, A.; Gross, R.A. Mild, Solvent-Free beta-Hydroxy Acid Polycondensations Catalyzed by Candida antarctica Lipase B. Biomacromolecules 2004, 5, 62–68. [Google Scholar] [CrossRef]
- Zinck, P. Synthetic Strategies for Biomedical Polyesters Specialties; Laskovski, A.N., Ed.; InTech: Rijeka, Croatia, 2011. Available online: http://www.intechopen.com/books/biomedical-engineering-trends-in-materials-science/synthetic-strategies-for-biomedical-polyesters-specialties/ (accessed on 15 September 2012).
- Odian, G. Principles of Polymerization; John Wiley & Sons: New York, NY, USA, 2004; p. 94. [Google Scholar]
- Ahmed, A.; Crawford, T.; Gould, S.; Ha, Y.S.; Hollrah, M.; Noor-E-Ain, Dickman, M.B.; Dussault, P.H. Synthesis of (R)- and (S)-10,16-dihydroxyhexadecanoic acid: Cutin stereochemistry and fungal activation. Phytochemistry 2003, 63, 47–52. [Google Scholar]
- Arrieta-Baez, D.; Cruz-Carrillo, M.; Gómez-Patiño, M.B.; Zepeda-Vallejo, L.G. Derivatives of 10,16-dihydroxyhexadecanoic acid isolated from tomato (Solanum lycopersicum) as potential material for aliphatic polyesters. Molecules 2011, 16, 4923–4936. [Google Scholar] [CrossRef]
- Namekawa, S.; Uyama, H.; Kobayashi, S. Enzymatic synthesis of polyesters from lactones, dicarboxylic acid divinyl esters, and glycols through combination of ring-opening polymerization and polycondensation. Biomacromolecules 2000, 1, 335–338. [Google Scholar] [CrossRef]
- Tulloch, A.P. Cutin acids: Synthesis and mass spectrometry of methyl 16-hydroxy-7-oxo-, 16-hydroxy-8-oxo-, 16-hydroxy-9-oxo-, 16-hydroxy-10-oxo- and 7,16-8,16-, 9,16- and 10,16-dihydroxyhexadecanoates. Lipids 1980, 15, 881–888. [Google Scholar] [CrossRef]
- Miletic, N.; Loos, K.; Gross, R.A. Enzymatic Polymerization of Polyester; Loos, K., Ed.; Biocatalysis in Polymer Chemistry: Weinheim, Germany, 2011; pp. 83–129. [Google Scholar]
- Kumar, A.; Garg, K.; Gross, R.A. Copolymerizations of ω-pentadecalactone and trimethylene carbonate by chemical and lipase catalysis. Macromolecules 2001, 34, 3527–3533. [Google Scholar] [CrossRef]
- Kumar, A.; Gross, R.A. Candida antarctica lipase B-catalyzed transesterification: New synthetic routes to copolyesters. J. Am. Chem. Soc. 2000, 122, 11767–11770. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1, 2, 3 and 4 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gómez-Patiño, M.B.; Cassani, J.; Jaramillo-Flores, M.E.; Zepeda-Vallejo, L.G.; Sandoval, G.; Jimenez-Estrada, M.; Arrieta-Baez, D. Oligomerization of 10,16-Dihydroxyhexadecanoic Acid and Methyl 10,16-Dihydroxyhexadecanoate Catalyzed by Lipases. Molecules 2013, 18, 9317-9333. https://doi.org/10.3390/molecules18089317
Gómez-Patiño MB, Cassani J, Jaramillo-Flores ME, Zepeda-Vallejo LG, Sandoval G, Jimenez-Estrada M, Arrieta-Baez D. Oligomerization of 10,16-Dihydroxyhexadecanoic Acid and Methyl 10,16-Dihydroxyhexadecanoate Catalyzed by Lipases. Molecules. 2013; 18(8):9317-9333. https://doi.org/10.3390/molecules18089317
Chicago/Turabian StyleGómez-Patiño, M. Beatriz, Julia Cassani, María Eugenia Jaramillo-Flores, L. Gerardo Zepeda-Vallejo, Georgina Sandoval, Manuel Jimenez-Estrada, and Daniel Arrieta-Baez. 2013. "Oligomerization of 10,16-Dihydroxyhexadecanoic Acid and Methyl 10,16-Dihydroxyhexadecanoate Catalyzed by Lipases" Molecules 18, no. 8: 9317-9333. https://doi.org/10.3390/molecules18089317