Eco-Friendly Synthesis and Antiproliferative Evaluation of Some Oxygen Substituted Diaryl Ketones
Abstract
:1. Introduction
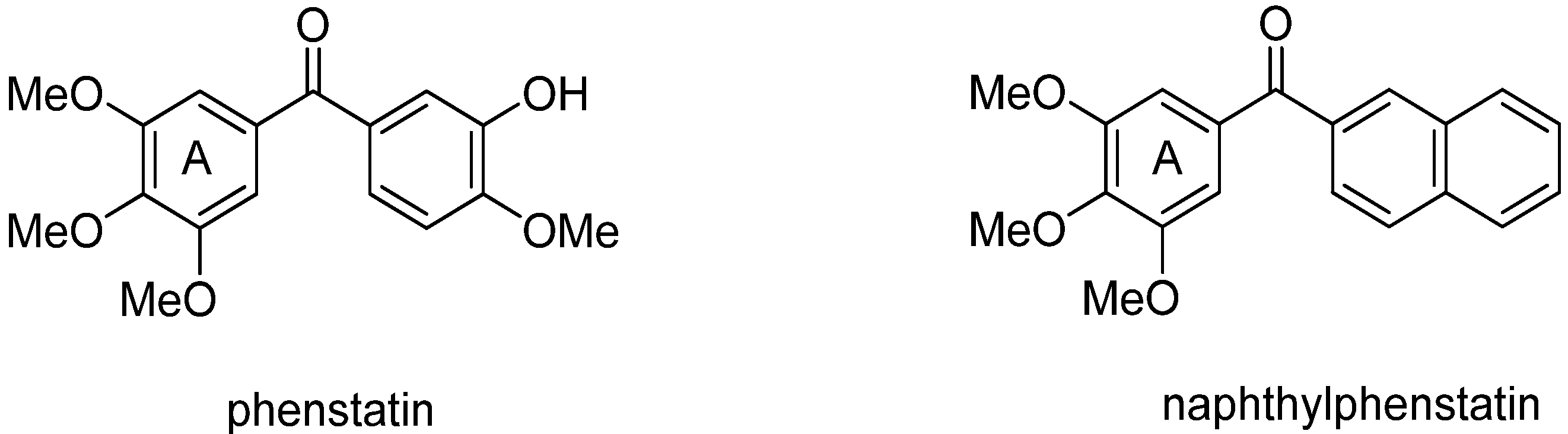
2. Results and Discussion
2.1. Chemistry

| Photoproduct | Substituents | Yield (%) a | ||||
|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | Method A b | Method B c | |
| 3 | H | H | H | H | 77 | 91 |
| 4 | Me | H | H | H | 52 | 80 |
| 5 | H | Me | H | H | 34 | 74 |
| 6 | H | H | Me | H | 58 | 82 |
| 7 | H | OMe | H | H | 38 | 74 |
| 8 | H | H | OMe | H | 59 | 79 |
| 9 | OMe | H | H | OMe | - | 70 |
| 10 | H | H | OMe | OMe | - | 70 |
| 11 | H | OMe | OH | H | - | 78 |
| 12 | H | OMe | OMe | OMe | - | 70 |
| 13 | H | OMe | OH | OMe | - | 70 |
| 14 | Me | H | H | H | 53 | 82 |
| 15 | H | Me | H | H | 41 | 69 |
| 16 | H | H | Me | H | 57 | 84 |
| 17 | H | OMe | H | H | 50 | 71 |
| 18 | H | H | OMe | H | 69 | 88 |
| 19 | OMe | H | H | OMe | - | 65 |
| 20 | H | H | OMe | OMe | - | 63 |
| 21 | H | H | OH | OMe | - | 73 |
| 22 | H | OMe | OMe | OMe | - | 60 |
| 23 | H | OMe | OH | OMe | - | 66 |
2.2. In Vitro Antiproliferative Activity of Diaryl Ketones 3–23 again Select Cancer Cell Lines

| EC50 ± SEM a (μM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| N° | R1 | R2 | R3 | R4 | T24 | DU-145 | MCF-7 | BALB/3T3 | MSI b |
| 3 | H | H | H | H | 186.4 ± 20.4 | 205.0 ± 21.5 | 189.8 ± 19.2 | 38.5 ± 5.5 | 0.20 |
| 4 | Me | H | H | H | 172.5 ± 13.2 | 172.5 ± 19.7 | 180.7 ± 15.8 | 183.9 ± 17.6 | 1.06 |
| 5 | H | Me | H | H | 202.3 ± 15.9 | 186.2 ± 17.8 | 225.7 ± 24.2 | 100.3 ± 9.5 | 0.49 |
| 6 | H | H | H | Me | 208.9 ± 19.5 | 100.8 ± 9.5 | 142.1 ± 15.3 | 78.3 ± 6.4 | 0.57 |
| 7 | H | OMe | H | H | 209.7 ± 20.5 | 166.1 ± 15.8 | 154.4 ± 12.3 | 117.6 ± 19.5 | 0.68 |
| 8 | H | H | OMe | H | 168.1 ± 12.2 | 170.2 ± 15.5 | 190.7 ± 17.5 | 85.2 ± 9.7 | 0.49 |
| 9 | OMe | H | H | OMe | 149.7 ± 18.4 | 130.3 ± 12.5 | 164.5 ± 13.8 | 43.7 ± 7.6 | 0.29 |
| 10 | H | H | OMe | OMe | 147.4 ± 19.2 | 140.0 ± 13.6 | 182.9 ± 17.7 | 35.4 ± 4.7 | 0.23 |
| 11 | H | OMe | OH | H | 154.5 ± 14.5 | 167.5 ± 15.9 | 176.7 ± 19.4 | 78.0 ± 9.3 | 0.47 |
| 12 | H | OMe | OMe | OMe | 3.6 ± 1.4 | 1.2 ± 0.6 | 53.6 ± 6.7 | 1.5 ± 0.4 | 0.57 |
| 13 | H | OMe | OH | OMe | >350 | 112.8 ± 10.7 | >350 | 19.1 ± 3.1 | - |
| 14 | Me | H | H | H | 143.3 ± 9.5 | 143.8 ± 17.7 | 152.3 ± 14.5 | 225.5 ± 25.4 | 1.54 |
| 15 | H | Me | H | H | 136.8 ± 11.7 | 147.4 ± 15.9 | 144.9 ± 12.7 | 142.4 ± 13.9 | 0.99 |
| 16 | H | H | Me | H | 134.6 ± 12.8 | 158.3 ± 13.7 | 133.8 ± 14.9 | 138.9 ± 15.2 | 0.98 |
| 17 | H | OMe | H | H | 133.3 ± 12.4 | 139.4 ± 11.3 | 127.9 ± 13.6 | 109.1 ± 11.1 | 0.82 |
| 18 | H | H | OMe | H | 126.8 ± 10.6 | 77.5 ± 6.5 | 124.9 ± 10.2 | 107.0 ± 12.3 | 1.03 |
| 19 | OMe | H | H | OMe | 129.8 ± 10.1 | 128.7 ± 17.2 | >310 | 100.3 ± 8.9 | 0.77 |
| 20 | H | H | OMe | OMe | 46.8 ± 5.1 | 61.8 ± 5.6 | 12.2 ± 3.8 | 2.8 ± 0.6 | 0.11 |
| 21 | H | H | OH | OMe | 86.6 ± 9.6 | 89.0 ± 7.3 | 15.7 ± 4.6 | 15.7 ± 3.9 | 0.45 |
| 22 | H | OMe | OMe | OMe | 13.5 ±1.9 | 5.9 ± 0.8 | 20.2 ± 4.5 | 25.1 ± 3.9 | 2.45 |
| 23 | H | OMe | OH | OMe | 112.0 ± 9.3 | 113.5 ± 9.5 | 130.2 ± 14.8 | 99.6 ± 7.5 | 0.84 |
| DOXc | - | - | - | - | 0.65 ± 0.07 | 0.42 ± 0.03 | 0.33 ± 0.05 | 0.19 ± 0.01 | 0.44 |
| MITd | - | - | - | - | 42.2 ± 5.8 | 14.3 ± 2.6 | 16.8 ± 2.9 | 27.3 ± 3.3 | 1.39 |
3. Experimental
3.1. General
3.2. Chemistry
General Procedure for Photoacylation of 1 and 2 with Substituted Benzaldehydes in the Absence of Benzene (Method A)
General Procedure for Photoacylation of 1 and 2 with Substituted Benzaldehydes in Benzene (Method B).
3.3. Antiproliferative Assay
3.3.1. Cell Lines and Culture Conditions
3.3.2. Cellular Assays
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Thompson, R.H. Naturally Occurring Quinones III, Recent Advances; Chapman and Hall: London, UK, 1987. [Google Scholar]
- Maruyama, K.; Naruta, Y. Syntheses of alpha- and beta-lapachones and their homologues by way of photochemical side chain introduction to quinone. Chem. Lett. 1977, 8, 847–850. [Google Scholar] [CrossRef]
- Uno, H. Allylation of 2-alkanoyl 1,4-quinones with allylsilanes and allylstannanes. Efficient synthesis of pyranonaphthoquinone antibiotics. J. Org. Chem. 1986, 51, 350–358. [Google Scholar] [CrossRef]
- Brimble, M.A.; Lynds, S.M. A short synthesis of deoxyfrenolicin. J. Chem. Soc. Perkin. Trans. 1 1994, 1, 493–496. [Google Scholar] [CrossRef]
- Kraus, G.A.; Maeda, H. A direct preparation of 1,4-benzodiazepines. The synthesis of medazepam and related compounds via a common intermediate. Tetrahedron Lett. 1994, 35, 9189–9190. [Google Scholar] [CrossRef]
- Waske, P.A.; Mattay, J.; Oelgemöller, M. Photoacylations of 2-substituted 1,4-naphthoquinones: A concise access to biologically active quinonoid compounds. Tetrahedron Lett. 2006, 47, 1329–1332. [Google Scholar] [CrossRef]
- Valderrama, J.A.; Pessoa-Mahana, D.; Tapia, R.A.; Rojas de Arias, A.; Nakayama, H.; Torres, S.; Miret, J.; Ferreira, M.E. Studies on quinones. Part 34: The reaction of styrene with activated 1,4-benzoquinones: Access to potential antiprotozoal pyranobenzoquinones. Tetrahedron 2001, 57, 8653–8658. [Google Scholar] [CrossRef]
- Valderrama, J.A.; Benites, J.; Cortés, M.; Pessoa-Mahana, D.; Prina, E.; Fournet, A. Studies on quinones. Part 35: Access to antiprotozoal active euryfurylquinones and hydroquinones. Tetrahedron 2002, 58, 881–886. [Google Scholar] [CrossRef]
- Valderrama, J.A.; Zamorano, C.; González, M.F.; Prina, E.; Fournet, A. Studies on quinones. Part 39: Synthesis and leishmanicidal activity of acylchloroquinones and hydroquinones. Bioorg. Med. Chem. 2005, 13, 4153–4159. [Google Scholar] [CrossRef]
- Valderrama, J.A.; González, M.F.; Pessoa-Mahana, D.; Tapia, R.A.; Fillion, H.; Pautet, F.; Rodríguez, J.A.; Theoduloz, C.; Schmeda-Hishmann, G. Studies on quinones. Part 41: Synthesis and cytotoxicity of isoquinoline-containing polycyclic quinones. Bioorg. Med. Chem. 2006, 14, 5003–5011. [Google Scholar] [CrossRef]
- Valderrama, J.A.; González, M.F.; Colonelli, P.; Vásquez, D. Design and synthesis of angucyclinone 5-aza analogues. Synlett 2006, 17, 2777–2780. [Google Scholar]
- Valderrama, J.A.; Vásquez, D. Design and synthesis of angucyclinone AB-pyrido[2,3-d]pyrimidine analogues. Tetrahedron Lett. 2008, 49, 703–706. [Google Scholar] [CrossRef]
- Vásquez, D.; Rodríguez, J.A.; Theoduloz, C.; Buc Calderon, P.; Valderrama, J.A. Studies on quinones. Part 46. Synthesis and in vitro antitumor evaluation of aminopyrimidoisoquinolinequinones. Eur. J. Med. Chem. 2010, 45, 5234–5242. [Google Scholar] [CrossRef]
- Valderrama, J.A.; Ibacache, A.; Rodriguez, J.A.; Theoduloz, C.; Benites, J. Studies on Quinones. Part 47. Synthesis of novel phenylaminophenanthridinequinones as potential antitumor agents. Eur. J. Med. Chem. 2011, 46, 3398–3409. [Google Scholar] [CrossRef]
- Vásquez, R.; Verrax, J.; Valderrama, J.A.; Buc Calderon, P. Aminopyrimidoisoquinolinequinone (APIQ) redox cycling is potentiated by ascorbate and induces oxidative stress leading to necrotic-like cancer cell death. Invest. New Drug. 2012, 30, 1003–1011. [Google Scholar] [CrossRef]
- Monsalve, F.A.; Valderrama, J.A.; Vásquez, D.; Ibacache, A.; Rodríguez, J.A.; González, D.R.; González, E. Inhibition of human topoisomerase I and activation of caspase-3 by aza-angucyclinones and arylaminopyrimido[4,5-c]isoquinoline-7,10-quinones. Int. J. Mol. Med. 2012, 30, 151–156. [Google Scholar]
- Kviecinski, M.R.; Pedrosa, R.C.; Felipe, K.B.; Farias, M.S.; Glorieux, C.; Valenzuela, M.; Sid, B.; Benites, J.; Valderrama, J.A.; Verrax, J.; et al. Inhibition of cell proliferation and migration by oxidative stress from ascorbate-driven juglone redox cycling in human bladder-derived T24 cells. Biochem. Biophys. Res. Commun. 2012, 421, 268–273. [Google Scholar] [CrossRef]
- Delgado, V.; Ibacache, A.; Theoduloz, C.; Valderrama, J.A. Synthesis and in vitro cytotoxic evaluation of aminoquinones structurally related to marine isoquinolinequinones. Molecules 2012, 17, 7042–7056. [Google Scholar] [CrossRef]
- Vásquez, D.; Theoduloz, C.; Benites, J.; Ríos, D.; Valderrama, J.A. Synthesis and antitumor evaluation of 6-arylsubstituted benzo[j]phenanthridine- and benzo[g]pyrimido[4,5-c]isoquinolinequinones. Molecules 2012, 17, 11616–11629. [Google Scholar] [CrossRef]
- Ríos, D.; Benites, J.; Valderrama, J.A.; Farias, M.; Pedrosa, R.C.; Buc Calderon, P.; Verrax, J. Biological evaluation of 3-acyl-2-arylamino-1,4-naphthoquinones as inhibitors of Hsp90 chaperoning function. Curr. Top. Med. Chem. 2012, 12, 2094–2102. [Google Scholar] [CrossRef]
- Naeimi, H.; Moradi, L. Microwave assisted direct ortho-acylation of phenol and naphthol derivatives by BF3.(C2H5)2O. Bull. Chem. Soc. Jpn. 2005, 78, 284–287. [Google Scholar] [CrossRef]
- Trost, B.M.; Saulnier, M.G. Regioselectivity in lithiation of t-butyldimethylsiloxy-3,5-dimethoxybenzene. A synthesis of the trimethyl ether of sophoraflavanone A. Tetrahedron Lett. 1985, 26, 123–126. [Google Scholar] [CrossRef]
- Crombie, L.; Jones, R.C.F.; Palmer, C.J. Synthesis of the insecticidal 1'-acetoxy-mammeins and surangin B. Tetrahedron Lett. 1985, 26, 2933–2936. [Google Scholar] [CrossRef]
- Duplais, C.; Bures, F.; Sapountzis, I.; Korn, T.J.; Cahiez, G.; Knochel, P. An efficient synthesis of diaryl ketones by iron-catalyzed arylation of aroyl cyanides. Angew. Chem. Int. Ed. Engl. 2004, 43, 2968–2970. [Google Scholar] [CrossRef]
- Satori, G.; Casnati, G.; Bigi, F.; Predieri, G. Ortho-coordinated acylation of phenol systems. J. Org. Chem. 1990, 55, 4371–4377. [Google Scholar] [CrossRef]
- Boyer, J.L.; Krum, J.E.; Myers, M.C.; Fazal, A.N.; Wigal, C.T. Synthetic utility and mechanistic implications of the Fries rearrangement of hydroquinone diesters in boron trifluoride complexes. J. Org. Chem. 2000, 65, 4712–4714. [Google Scholar] [CrossRef]
- Klinger, H.; Kolvenbach, W. Die bildung von acetohydrochinon aus acetaldehyd und benzochinon im sonnenlicht. Ber. Dtsch. Chem. Ges. 1898, 31, 1214–1216. [Google Scholar] [CrossRef]
- Patai, S. The Chemistry of the Quinoid Compounds, Part 1; John Wiley and Sons: New York, NY, USA, 1974; Vol. 1, p. 503. [Google Scholar]
- Kraus, G.A.; Liu, P. Benzophenone-mediated conjugate additions of aromatic aldehydes to quinones. Tetrahedron Lett. 1994, 35, 7723–7726. [Google Scholar]
- Oelgemöller, M.; Schiel, C.; Fröhlich, R.; Mattay, J. The photo-Friedel-Crafts acylation of 1,4-naphthoquinones. Eur. J. Org. Chem. 2002, 15, 2465–2474. [Google Scholar]
- Oelgemöller, M.; Jung, C.; Ortner, J.; Mattay, J.; Schiel, C.; Zimmermann, E. “Green photochemistry” with moderately concentrated sunlight. Spectrum 2005, 18, 28–33. [Google Scholar]
- Murphy, B.; Goodrich, P.; Hardacre, C.; Oelgemöller, M. Green photochemistry: Photo-Friedel–Crafts acylations of 1,4-naphthoquinone in room temperature ionic liquids. Green Chem. 2009, 11, 1867–1870. [Google Scholar] [CrossRef]
- Benites, J.; Ríos, D.; Díaz, P.; Valderrama, J.A. The solar-chemical photo-Friedel–Crafts heteroacylation of 1,4-quinones. Tetrahedron Lett. 2011, 52, 609–611. [Google Scholar] [CrossRef]
- De Leon, F.; Kalagara, S.; Navarro, A.A.; Mito, S. Synthesis of 6-acyl-5,8-quinolinediols by Photo-Friedel–Crafts acylation using sunlight. Tetrahedron Lett. 2013, 54, 3147–3149. [Google Scholar] [CrossRef]
- Pettit, G.R.; Toki, B.; Herald, D.L.; Verdier-Pinard, P.; Boyd, M.R.; Hamel, E.; Pettit, R.K. Antineoplastic agents. 379. Synthesis of phenstatin phosphate. J. Med. Chem. 1998, 41, 1688–1698. [Google Scholar] [CrossRef]
- Alvarez, C.; Alvarez, R.; Corchete, P.; Pérez-Melero, C.; Peláez, R.; Medarde, M. Synthesis and biological activity of naphthalene analogues of phenstatins: Naphthylphenstatins. Bioorg. Med. Chem. Lett. 2007, 17, 3417–3420. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Cushman, M.; Nagarathnam, D.; Gopal, D.; Chakraborti, A.K.; Lin, C.M.; Hamel, E. Synthesis and evaluation of stilbene and dihydrostilbene derivatives as potential anticancer agents that inhibit tubulin polymerization. J. Med. Chem. 1991, 34, 2579–2588. [Google Scholar] [CrossRef]
- Pettit, G.R.; Grealish, M.P.; Jung, M.K.; Hamel, E.; Pettit, R.K.; Chapuis, J.C.; Schmidt, J.M. Antineoplastic agents. 465. Structural modification of resveratrol: sodium. Resverastatin phosphate. J. Med. Chem. 2002, 45, 2534–2542. [Google Scholar] [CrossRef]
- Liou, J.P.; Chang, Y.L.; Kuo, F.M.; Chang, C.W.; Tseng, H.Y.; Wang, C.C.; Yang, Y.N.; Chang, J.Y.; Lee, S.J.; Hsieh, H.P. Concise synthesis and structure-activity relationships of combretastatin A-4 analogues, 1-aroylindoles and 3-aroylindoles, as novel classes of potent antitubulin agents. J. Med. Chem. 2004, 47, 4247–4257. [Google Scholar] [CrossRef]
- Tron, G.C.; Pirali, T.; Sorba, G.; Pagliai, F.; Busacca, S.; Genazzani, A.A. Medicinal chemistry of combretastatin A4: Present and future directions. J. Med. Chem. 2006, 49, 3033–3044. [Google Scholar] [CrossRef]
- Romagnoli, R.; Baraldi, P.G.; Carrion, M.D.; Lopez Cara, C.; Preti, D.; Fruttarolo, F.; Pavani, M. G.; Tabrizi, M.A.; Tolomeo, M.; Grimaudo, S.; et al. Synthesis and biological evaluation of 2- and 3-aminobenzo[b]thiophene derivatives as antimitotic agents and inhibitors of tubulin polymerization. J. Med. Chem. 2007, 50, 2273–2277. [Google Scholar] [CrossRef]
- Hu, L.; Jiang, J.D.; Qu, J.; Li, Y.; Jin, J.; Li, Z.R.; Boykin, D.W. Novel potent antimitotic heterocyclic ketones: Synthesis, Antiproliferative activity, and structure–activity relationships. Bioorg. Med. Chem. Lett. 2007, 17, 3613–3617. [Google Scholar] [CrossRef]
- Ty, N.; Kaffy, J.; Arrault, A.; Thoret, S.; Pontikis, R.; Dubois, J.; Morin-Allory, L.; Florent, J.C. Synthesis and biological evaluation of cis-locked vinylogous combretastatin-A4 analogues: Derivatives with a cyclopropyl-vinyl or a cyclopropyl-amide bridge. Bioorg. Med. Chem. Lett. 2009, 19, 1318–1322. [Google Scholar] [CrossRef]
- Ghinet, A.; Rigo, B.; Hénichart, J.P.; Le Broc-Ryckewaert, D.; Pommery, J.; Pommery, N.; Thuru, X.; Quesnel, B.; Gautret, P. Synthesis and biological evaluation of phenstatin metabolites. Bioorgn. Med. Chem. 2011, 19, 6042–6054. [Google Scholar] [CrossRef]
- Bogert, M.T.; Howells, H.P. The chemistry of the acyl para-quinones. A contribution to the solution of the “Pechmann dyes” problem. J. Am. Chem. Soc. 1930, 52, 837–850. [Google Scholar] [CrossRef]
- Abdulla, K.A.; Abdul-Rahman, A.L.; Al-Hamdany, R.; Al-Saigh, Z. Preparation and light induced reactions of substituted 1,4-benzoquinones. J. Prakt. Chem. 1982, 34, 498–504. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Arenas, P.; Peña, A.; Ríos, D.; Benites, J.; Muccioli, G.G.; Calderon, P.B.; Valderrama, J.A. Eco-Friendly Synthesis and Antiproliferative Evaluation of Some Oxygen Substituted Diaryl Ketones. Molecules 2013, 18, 9818-9832. https://doi.org/10.3390/molecules18089818
Arenas P, Peña A, Ríos D, Benites J, Muccioli GG, Calderon PB, Valderrama JA. Eco-Friendly Synthesis and Antiproliferative Evaluation of Some Oxygen Substituted Diaryl Ketones. Molecules. 2013; 18(8):9818-9832. https://doi.org/10.3390/molecules18089818
Chicago/Turabian StyleArenas, Paola, Andrés Peña, David Ríos, Julio Benites, Giulio G. Muccioli, Pedro Buc Calderon, and Jaime A. Valderrama. 2013. "Eco-Friendly Synthesis and Antiproliferative Evaluation of Some Oxygen Substituted Diaryl Ketones" Molecules 18, no. 8: 9818-9832. https://doi.org/10.3390/molecules18089818





