Sex Differences in the Hepatic Cholesterol Sensing Mechanisms in Mice
Abstract
:1. Introduction
2. Results and Discussion
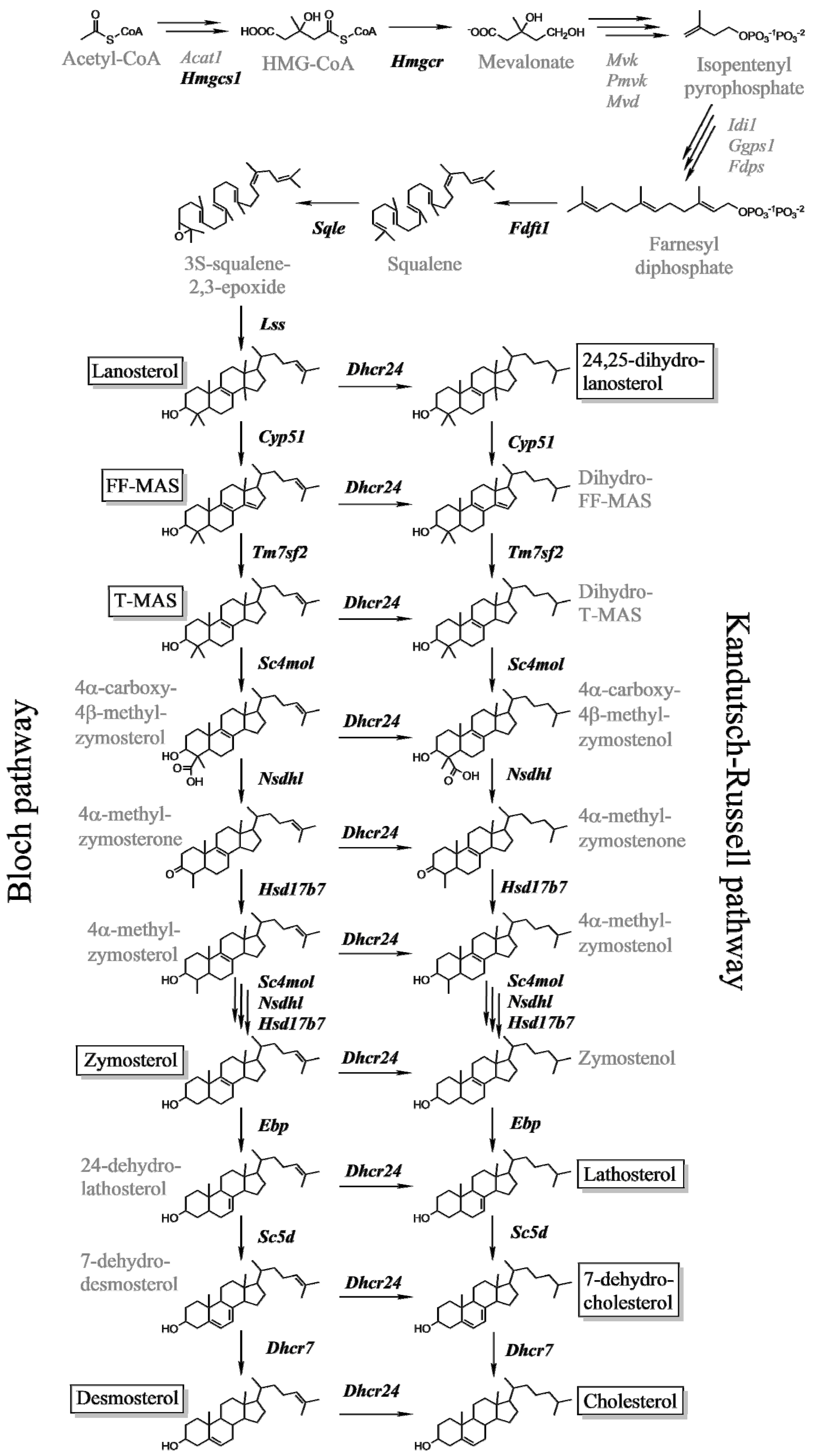
2.1. Expression of Cholesterogenic Genes in Livers of Male and Female Mice
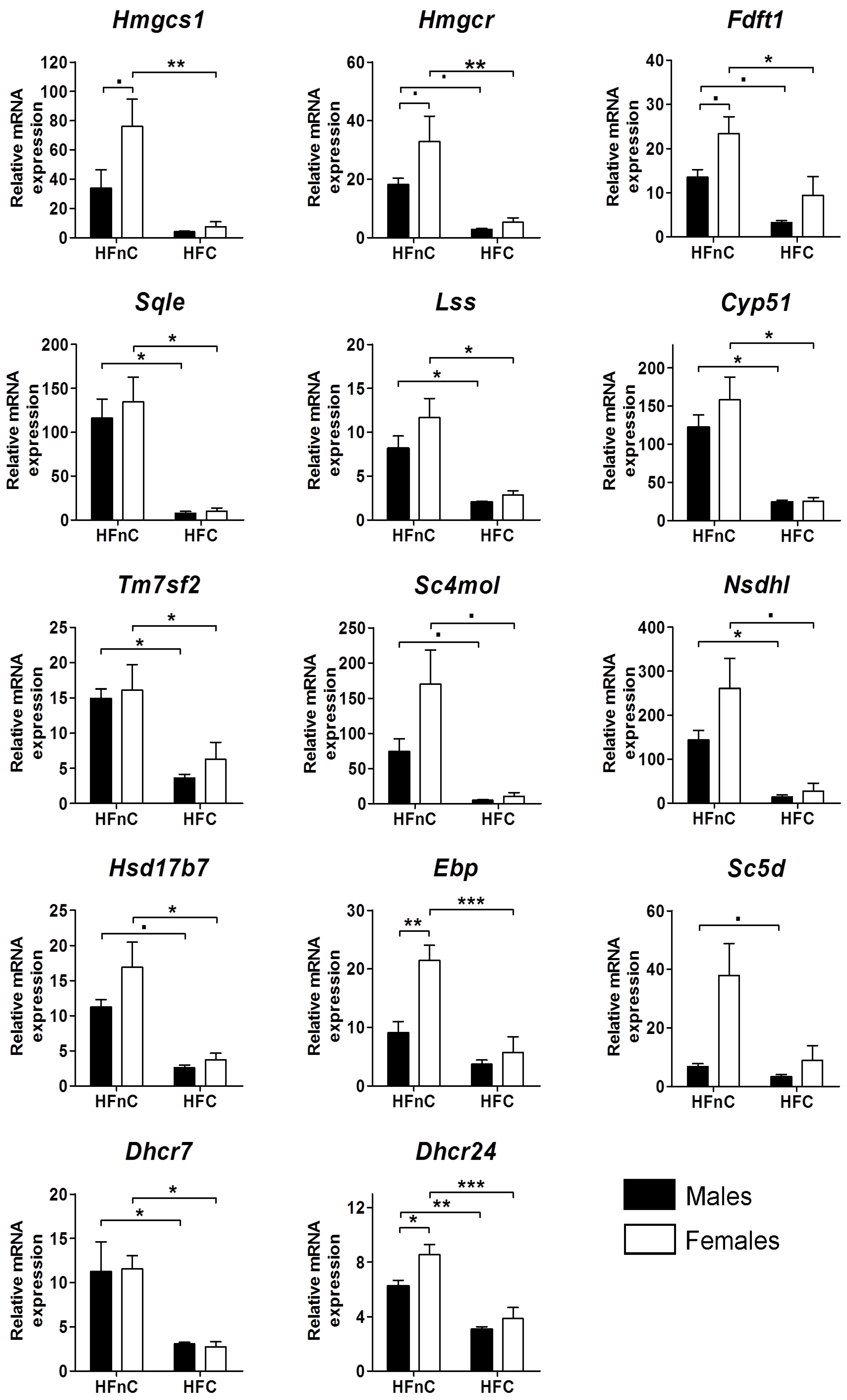
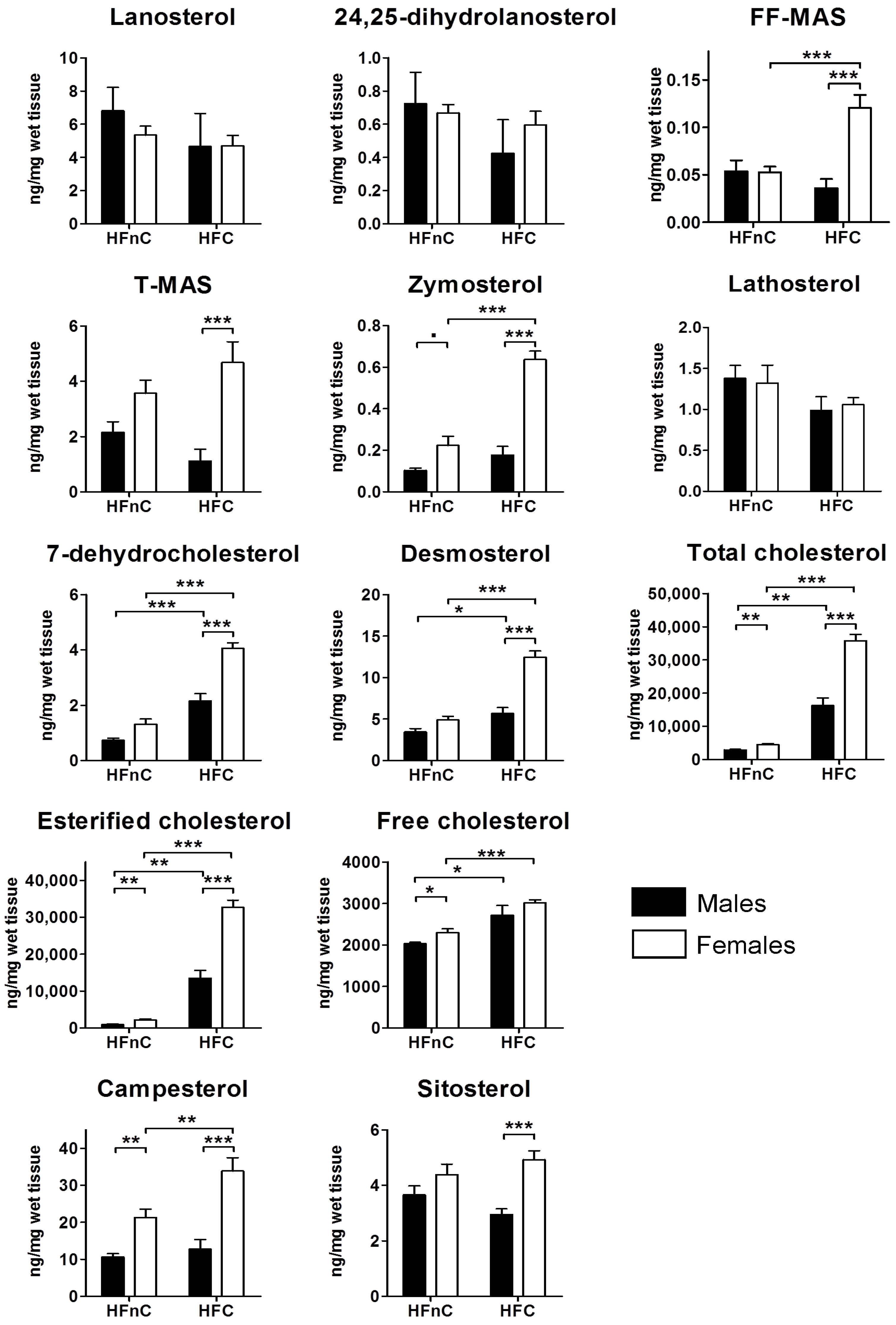
2.2. Hepatic Metabolites of Cholesterol Synthesis
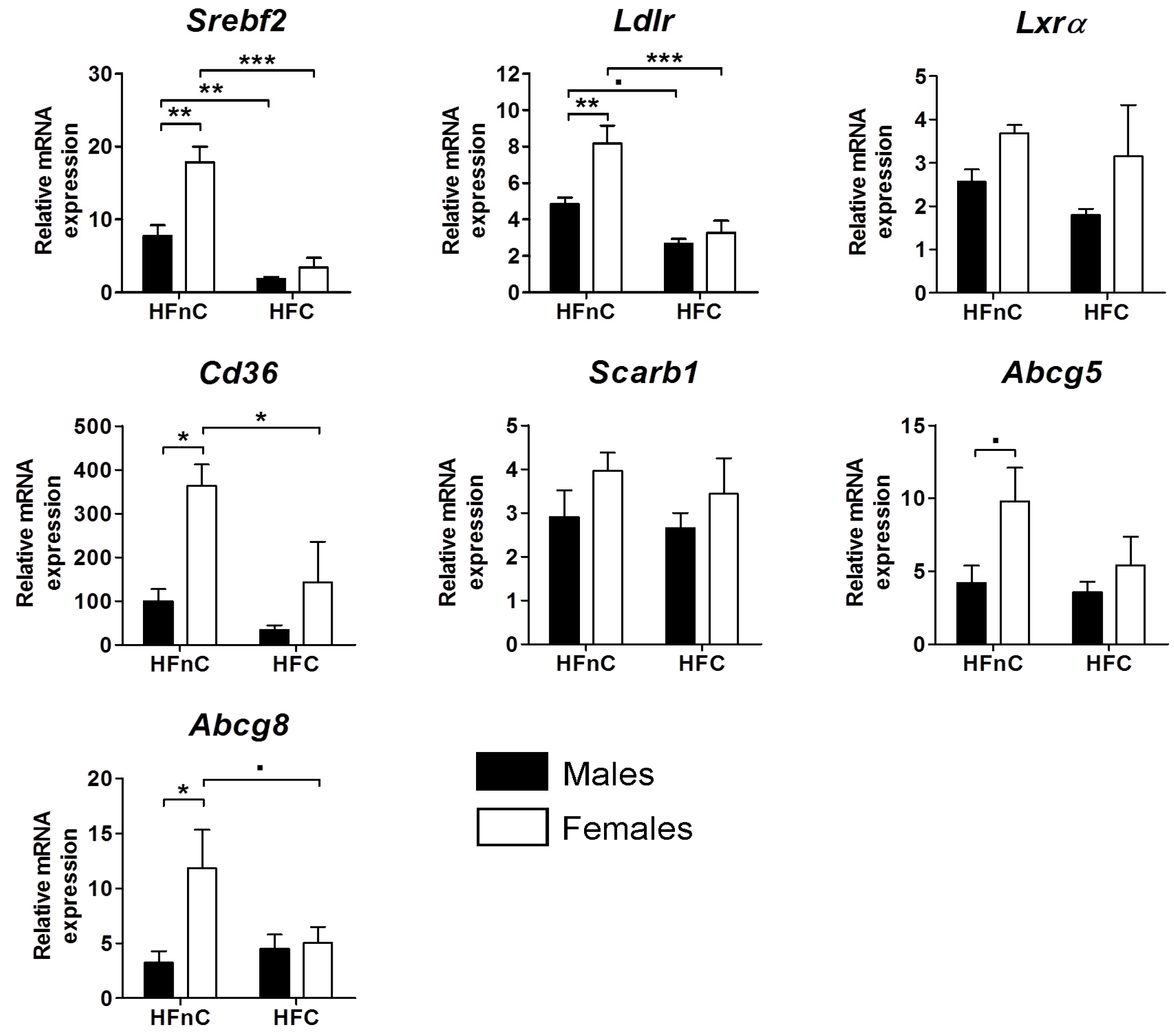
2.3. Expression of Hepatic Genes of Cholesterol Regulation and Transport
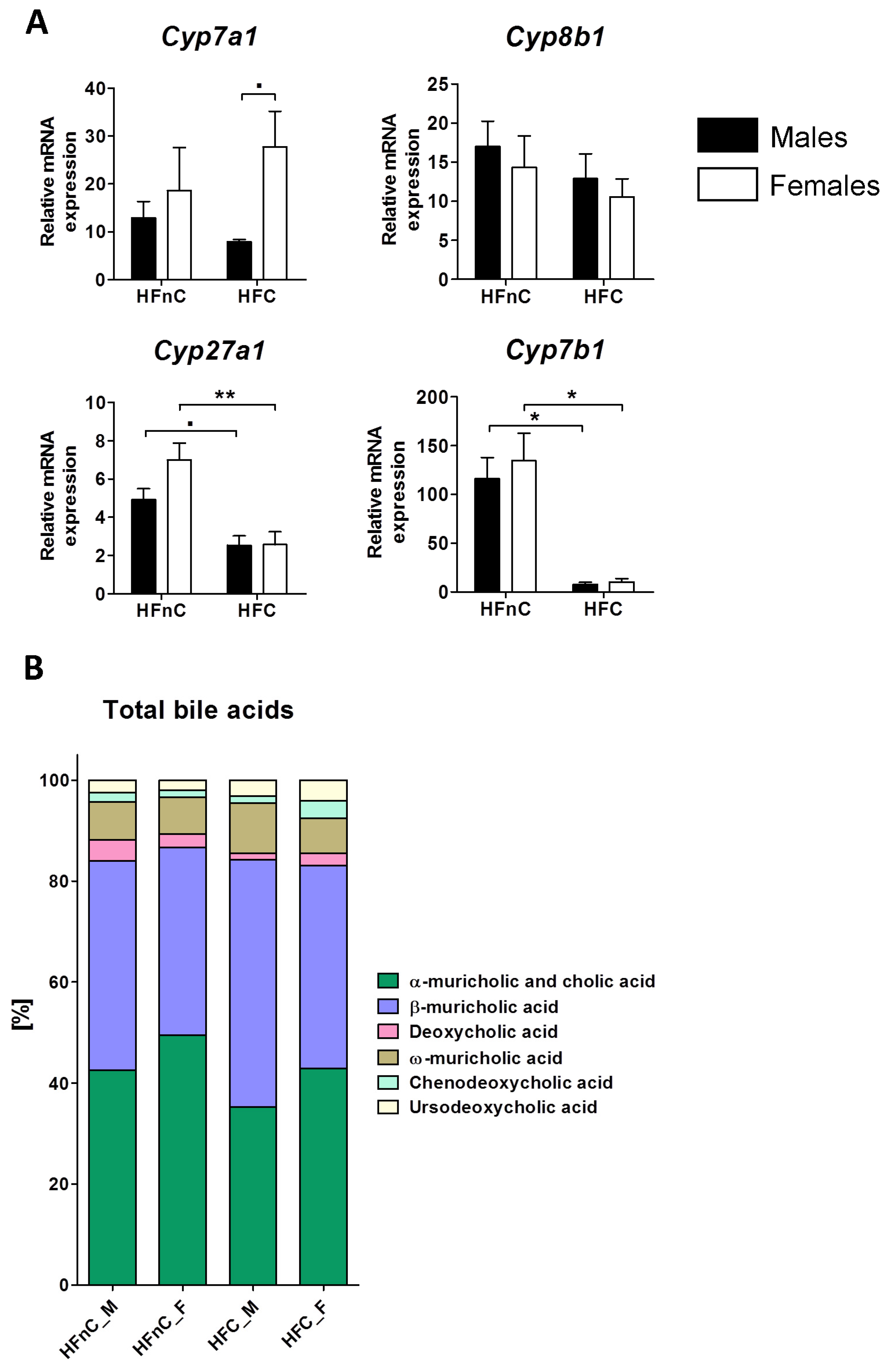
2.4. Bile Acid Synthesis Genes and Metabolites
2.5. Discussion
2.5.1. Female-Biased Expression of Cholesterogenic Genes is Diminished upon Addition of Dietary Cholesterol
2.5.2. Dietary Cholesterol in High-Fat Diet Down-Regulates the Entire Cholesterol Synthesis Pathway
2.5.3. Female Mice Have Higher Hepatic Pool of Cholesterol and Post-Lanosterol Synthesis Intermediates
2.5.4. Dietary Cholesterol or Sex Do Not Impact Gallbladder Bile Acid Profile
3. Experimental
3.1. Animals and Diets
3.2. Sample Collection
3.3. RNA Isolation and cDNA Synthesis
3.4. Real-Time Quantitative PCR
3.5. Total Sterol Extraction and GC-MS Analysis
3.6. Total Bile Acids Extraction and GC-MS Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Ramasubbu, K.; Gurm, H.; Litaker, D. Gender bias in clinical trials: do double standards still apply? J. Womens Health Gend. Based Med. 2001, 10, 757–764. [Google Scholar]
- Pilote, L.; Dasgupta, K.; Guru, V.; Humphries, K.H.; McGrath, J.; Norris, C.; Rabi, D.; Tremblay, J.; Alamian, A.; Barnett, T.; et al. A comprehensive view of sex-specific issues related to cardiovascular disease. Can. Med. Assn. J. 2007, 176, S1–S44. [Google Scholar]
- Kautzky-Willer, A.; Handisurya, A. Metabolic diseases and associated complications: Sex and gender matter! Eur. J. Clin. Invest. 2009, 39, 631–648. [Google Scholar]
- Lleo, A.; Battezzati, P.M.; Selmi, C.; Gershwin, M.E.; Podda, M. Is autoimmunity a matter of sex? Autoimmun. Rev. 2008, 7, 626–630. [Google Scholar] [CrossRef]
- Trapani, L.; Pallottini, V. Age-related hypercholesterolemia and hmg-coa reductase dysregulation: sex does matter (a gender perspective). Curr. Gerontol. Geriatr. Res. 2010, 2010, 420139. [Google Scholar]
- Nagoshi, S. Sex- or gender-specific medicine in hepatology. Hepatol. Res. 2008, 38, 219–224. [Google Scholar] [CrossRef]
- Rozman, D.; Monostory, K. Perspectives of the non-statin hypolipidemic agents. Pharmacol. Ther. 2010, 127, 19–40. [Google Scholar] [CrossRef]
- Thacker, H.L. Sex, Statins, and diabetes. Cleve. Clin. J. Med. 2013, 80, 187–187. [Google Scholar] [CrossRef]
- Fon Tacer, K.; Rozman, D. Nonalcoholic Fatty liver disease: focus on lipoprotein and lipid deregulation. J. Lipids 2011, 2011, 1–14. [Google Scholar]
- Lorbek, G.; Rozman, D. Cholesterol and Inflammation at the Crossroads of Non-Alcoholic Fatty Liver Disease (NAFLD) and Atherogenesis. In Atherogenesis; Parthasarathy, S., Ed.; InTech: Rijeka, Croatia, 2012; pp. 281–304. [Google Scholar]
- Zhang, H.; He, S.M.; Sun, J.; Wang, C.; Jiang, Y.F.; Gu, Q.; Feng, X.W.; Du, B.; Wang, W.; Shi, X.D.; et al. Prevalence and etiology of abnormal liver tests in an adult population in Jilin, China. Int. J. Med. Sci. 2011, 8, 254–262. [Google Scholar]
- Pinidiyapathirage, M.J.; Dassanayake, A.S.; Rajindrajith, S.; Kalubowila, U.; Kato, N.; Wickremasinghe, A.R.; de Silva, H.J. Non-alcoholic fatty liver disease in a rural, physically active, low income population in Sri Lanka. BMC Res. Notes 2011, 4, 513. [Google Scholar] [CrossRef]
- Denzer, C.; Thiere, D.; Muche, R.; Koenig, W.; Mayer, H.; Kratzer, W.; Wabitsch, M. Gender-specific prevalences of fatty liver in obese children and adolescents: roles of body fat distribution, sex steroids, and insulin resistance. J. Clin. Endocrinol. Metab. 2009, 94, 3872–3881. [Google Scholar] [CrossRef]
- Fernandes, M.T.; Ferraro, A.A.; de Azevedo, R.A.; Fagundes Neto, U. Metabolic differences between male and female adolescents with non-alcoholic fatty liver disease, As detected by ultrasound. Acta Paediatr. 2010, 99, 1218–1223. [Google Scholar] [CrossRef]
- Ayonrinde, O.T.; Olynyk, J.K.; Beilin, L.J.; Mori, T.A.; Pennell, C.E.; de Klerk, N.; Oddy, W.H.; Shipman, P.; Adams, L.A. Gender-specific differences in adipose distribution and adipocytokines influence adolescent nonalcoholic fatty liver disease. Hepatology 2011, 53, 800–809. [Google Scholar] [CrossRef]
- Kirsch, R.; Clarkson, V.; Shephard, E.G.; Marais, D.A.; Jaffer, M.A.; Woodburne, V.E.; Kirsch, R.E.; Hall Pde, L. Rodent nutritional model of non-alcoholic steatohepatitis: Species, Strain and sex difference studies. J. Gastroenterol. Hepatol. 2003, 18, 1272–1282. [Google Scholar] [CrossRef]
- Spruss, A.; Henkel, J.; Kanuri, G.; Blank, D.; Puschel, G.P.; Bischoff, S.C.; Bergheim, I. Female mice are more susceptible to nonalcoholic fatty liver disease: Sex-specific regulation of the hepatic AMP-activated protein kinase-plasminogen activator inhibitor 1 cascade, But not the hepatic endotoxin response. Mol. Med. 2012, 18, 1346–1355. [Google Scholar]
- Comhair, T.M.; Garcia Caraballo, S.C.; Dejong, C.H.; Lamers, W.H.; Kohler, S.E. Dietary cholesterol, Female gender and n-3 fatty acid deficiency are more important factors in the development of non-alcoholic fatty liver disease than the saturation index of the fat. Nutr. Metab. (Lond) 2011, 8, 4. [Google Scholar] [CrossRef]
- Larter, C.Z.; Yeh, M.M. Animal models of NASH: getting both pathology and metabolic context right. J. Gastroenterol. Hepatol. 2008, 23, 1635–1648. [Google Scholar] [CrossRef]
- Dietschy, J.M.; Turley, S.D. Control of cholesterol turnover in the mouse. J. Biol. Chem. 2002, 277, 3801–3804. [Google Scholar] [CrossRef]
- Jolley, C.D.; Dietschy, J.M.; Turley, S.D. Genetic differences in cholesterol absorption in 129/Sv and C57BL/6 mice: effect on cholesterol responsiveness. Am. J. Physiol. 1999, 276, G1117–G1124. [Google Scholar]
- Carlson, S.E.; Mitchell, A.D.; Carter, M.L.; Goldfarb, S. Evidence that physiologic levels of circulating estrogens and neonatal sex-imprinting modify postpubertal hepatic microsomal 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. Biochim. Biophys. Acta 1980, 633, 154–161. [Google Scholar] [CrossRef]
- Kojima, M.; Degawa, M. Gender-related difference in altered gene expression of a sterol regulatory element binding protein, SREBP-2, by lead nitrate in rats: correlation with development of hypercholesterolemia. J. Appl. Toxicol. 2006, 26, 381–384. [Google Scholar] [CrossRef]
- Hewitt, K.N.; Boon, W.C.; Murata, Y.; Jones, M.E.; Simpson, E.R. The aromatase knockout mouse presents with a sexually dimorphic disruption to cholesterol homeostasis. Endocrinology 2003, 144, 3895–3903. [Google Scholar] [CrossRef]
- De Marinis, E.; Martini, C.; Trentalance, A.; Pallottini, V. Sex differences in hepatic regulation of cholesterol homeostasis. J. Endocrinol. 2008, 198, 635–643. [Google Scholar] [CrossRef]
- Conforto, T.L.; Waxman, D.J. Sex-specific mouse liver gene expression: genome-wide analysis of developmental changes from pre-pubertal period to young adulthood. Biol. Sex Differ. 2012, 3, 9. [Google Scholar] [CrossRef]
- Yang, X.; Schadt, E.E.; Wang, S.; Wang, H.; Arnold, A.P.; Ingram-Drake, L.; Drake, T.A.; Lusis, A.J. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome. Res. 2006, 16, 995–1004. [Google Scholar] [CrossRef]
- Gatti, D.M.; Zhao, N.; Chesler, E.J.; Bradford, B.U.; Shabalin, A.A.; Yordanova, R.; Lu, L.; Rusyn, I. Sex-specific gene expression in the BXD mouse liver. Physiol. Genomics. 2010, 42, 456–468. [Google Scholar]
- Maxwell, K.N.; Soccio, R.E.; Duncan, E.M.; Sehayek, E.; Breslow, J.L. Novel putative SREBP and LXR target genes identified by microarray analysis in liver of cholesterol-fed mice. J. Lipid. Res. 2003, 44, 2109–2119. [Google Scholar] [CrossRef]
- Zhang, Y.; Klein, K.; Sugathan, A.; Nassery, N.; Dombkowski, A.; Zanger, U.M.; Waxman, D.J. Transcriptional profiling of human liver identifies sex-biased genes associated with polygenic dyslipidemia and coronary artery disease. PLoS One 2011, 6, e23506. [Google Scholar]
- Acimovic, J.; Rozman, D. Steroidal triterpenes of cholesterol synthesis. Molecules 2013, 18, 4002–4017. [Google Scholar] [CrossRef]
- Goldstein, J.L.; DeBose-Boyd, R.A.; Brown, M.S. Protein sensors for membrane sterols. Cell 2006, 124, 35–46. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 431–438. [Google Scholar] [CrossRef]
- Zhao, C.; Dahlman-Wright, K. Liver X receptor in cholesterol metabolism. J. Endocrinol. 2010, 204, 233–240. [Google Scholar] [CrossRef]
- Repa, J.J.; Berge, K.E.; Pomajzl, C.; Richardson, J.A.; Hobbs, H.; Mangelsdorf, D.J. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J. Biol. Chem. 2002, 277, 18793–18800. [Google Scholar]
- Wiersma, H.; Gatti, A.; Nijstad, N.; Oude Elferink, R.P.; Kuipers, F.; Tietge, U.J. Scavenger receptor class B type I mediates biliary cholesterol secretion independent of ATP-binding cassette transporter g5/g8 in mice. Hepatology 2009, 50, 1263–1272. [Google Scholar]
- Stahlberg, N.; Rico-Bautista, E.; Fisher, R.M.; Wu, X.; Cheung, L.; Flores-Morales, A.; Tybring, G.; Norstedt, G.; Tollet-Egnell, P. Female-predominant expression of fatty acid translocase/CD36 in rat and human liver. Endocrinology 2004, 145, 1972–1979. [Google Scholar]
- Henkel, A.S.; Anderson, K.A.; Dewey, A.M.; Kavesh, M.H.; Green, R.M. A chronic high-cholesterol diet paradoxically suppresses hepatic CYP7A1 expression in FVB/NJ mice. J. Lipid. Res. 2011, 52, 289–298. [Google Scholar]
- Zaczek, D.; Hammond, J.; Suen, L.; Wandji, S.; Service, D.; Bartke, A.; Chandrashekar, V.; Coschigano, K.; Kopchick, J. Impact of growth hormone resistance on female reproductive function: new insights from growth hormone receptor knockout mice. Biol. Reprod. 2002, 67, 1115–1124. [Google Scholar]
- Horton, J.D.; Shah, N.A.; Warrington, J.A.; Anderson, N.N.; Park, S.W.; Brown, M.S.; Goldstein, J.L. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. USA 2003, 100, 12027–12032. [Google Scholar]
- Dietschy, J.M.; Turley, S.D.; Spady, D.K. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J. Lipid. Res. 1993, 34, 1637–1659. [Google Scholar]
- Boone, L.R.; Brooks, P.A.; Niesen, M.I.; Ness, G.C. Mechanism of resistance to dietary cholesterol. J. Lipids 2011, 2011, 101242. [Google Scholar]
- Jo, Y.; Debose-Boyd, R.A. Control of cholesterol synthesis through regulated ER-associated degradation of HMG CoA reductase. Crit. Rev. Biochem. Molec. Biol. 2010, 45, 185–198. [Google Scholar]
- Gill, S.; Stevenson, J.; Kristiana, I.; Brown, A.J. Cholesterol-dependent degradation of squalene monooxygenase, a control point in cholesterol synthesis beyond HMG-CoA reductase. Cell Metab. 2011, 13, 260–273. [Google Scholar] [CrossRef]
- Thomas, S.; Fell, D.A. The role of multiple enzyme activation in metabolic flux control. Adv. Enzym. Regul. 1998, 38, 65–85. [Google Scholar] [CrossRef]
- Turley, S.D.; Schwarz, M.; Spady, D.K.; Dietschy, J.M. Gender-related differences in bile acid and sterol metabolism in outbred CD-1 mice fed low- and high-cholesterol diets. Hepatology 1998, 28, 1088–1094. [Google Scholar] [CrossRef]
- Plat, J.; de Jong, A.; Volger, O.L.; Princen, H.M.; Mensink, R.P. Preferential campesterol incorporation into various tissues in apolipoprotein E*3-Leiden mice consuming plant sterols or stanols. Metabolism 2008, 57, 1241–1247. [Google Scholar] [CrossRef]
- Cheema, S.K.; Cikaluk, D.; Agellon, L.B. Dietary fats modulate the regulatory potential of dietary cholesterol on cholesterol 7 alpha-hydroxylase gene expression. J. Lipid. Res. 1997, 38, 315–323. [Google Scholar]
- Schaffer, R.; Sniegoski, L.T.; Welch, M.J.; White, V.E.; Cohen, A.; Hertz, H.S.; Mandel, J.; Paule, R.C.; Svensson, L.; Bjorkhem, I.; et al. Comparison of two isotope dilution/mass spectrometric methods for determination of total serum cholesterol. Clin. Chem. 1982, 28, 5–8. [Google Scholar]
- Sharpe, L.J.; Brown, A.J. Controlling cholesterol synthesis beyond 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR). J. Biol. Chem. 2013. [Google Scholar] [CrossRef]
- Fitzky, B.U.; Moebius, F.F.; Asaoka, H.; Waage-Baudet, H.; Xu, L.; Xu, G.; Maeda, N.; Kluckman, K.; Hiller, S.; Yu, H.; et al. 7-Dehydrocholesterol-dependent proteolysis of HMG-CoA reductase suppresses sterol biosynthesis in a mouse model of Smith-Lemli-Opitz/RSH syndrome. J. Clin. Invest. 2001, 108, 905–915. [Google Scholar]
- Yang, C.; McDonald, J.G.; Patel, A.; Zhang, Y.; Umetani, M.; Xu, F.; Westover, E.J.; Covey, D.F.; Mangelsdorf, D.J.; Cohen, J.C.; et al. Sterol intermediates from cholesterol biosynthetic pathway as liver X receptor ligands. J. Biol. Chem. 2006, 281, 27816–27826. [Google Scholar] [CrossRef]
- Belic, A.; Pompon, D.; Monostory, K.; Kelly, D.; Kelly, S.; Rozman, D. An algorithm for rapid computational construction of metabolic networks: a cholesterol biosynthesis example. Comput. Biol. Med. 2013, 43, 471–480. [Google Scholar] [CrossRef]
- Kowdley, K.V. Ursodeoxycholic acid therapy in hepatobiliary disease. Am. J. Med. 2000, 108, 481–486. [Google Scholar] [CrossRef]
- Nie, B.; Park, H.M.; Kazantzis, M.; Lin, M.; Henkin, A.; Ng, S.; Song, S.; Chen, Y.; Tran, H.; Lai, R.; et al. Specific bile acids inhibit hepatic fatty acid uptake in mice. Hepatology 2012, 56, 1300–1310. [Google Scholar] [CrossRef]
- Hofmann, A.F. The enterohepatic circulation of bile acids in mammals: Form and functions. Front. Biosci. 2009, 14, 2584–2598. [Google Scholar]
- Kosir, R.; Acimovic, J.; Golicnik, M.; Perse, M.; Majdic, G.; Fink, M.; Rozman, D. Determination of reference genes for circadian studies in different tissues and mouse strains. BMC Mol. Biol. 2010, 11, 60. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome. Biol. 2007, 8, R19. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome. Biol. 2002, 3, R0034.1–R0034.11. [Google Scholar]
- Acimovic, J.; Lovgren-Sandblom, A.; Monostory, K.; Rozman, D.; Golicnik, M.; Lutjohann, D.; Bjorkhem, I. Combined gas chromatographic/mass spectrometric analysis of cholesterol precursors and plant sterols in cultured cells. J. Chromatogr. B 2009, 877, 2081–2086. [Google Scholar] [CrossRef]
- Min, H.K.; Kapoor, A.; Fuchs, M.; Mirshahi, F.; Zhou, H.; Maher, J.; Kellum, J.; Warnick, R.; Contos, M.J.; Sanyal, A.J. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012, 15, 665–674. [Google Scholar] [CrossRef]
- Wouters, K.; van Gorp, P.J.; Bieghs, V.; Gijbels, M.J.; Duimel, H.; Lutjohann, D.; Kerksiek, A.; van Kruchten, R.; Maeda, N.; Staels, B.; et al. Dietary cholesterol, Rather than liver steatosis, Leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology 2008, 48, 474–486. [Google Scholar] [CrossRef]
- Mari, M.; Caballero, F.; Colell, A.; Morales, A.; Caballeria, J.; Fernandez, A.; Enrich, C.; Fernandez-Checa, J.C.; Garcia-Ruiz, C. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 2006, 4, 185–198. [Google Scholar] [CrossRef]
- Rozman, D.; Waterman, M.R. Lanosterol 14alpha-demethylase (CYP51) and spermatogenesis. Drug Metab. Dispos. 1998, 26, 1199–1201. [Google Scholar]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lorbek, G.; Perše, M.; Horvat, S.; Björkhem, I.; Rozman, D. Sex Differences in the Hepatic Cholesterol Sensing Mechanisms in Mice. Molecules 2013, 18, 11067-11085. https://doi.org/10.3390/molecules180911067
Lorbek G, Perše M, Horvat S, Björkhem I, Rozman D. Sex Differences in the Hepatic Cholesterol Sensing Mechanisms in Mice. Molecules. 2013; 18(9):11067-11085. https://doi.org/10.3390/molecules180911067
Chicago/Turabian StyleLorbek, Gregor, Martina Perše, Simon Horvat, Ingemar Björkhem, and Damjana Rozman. 2013. "Sex Differences in the Hepatic Cholesterol Sensing Mechanisms in Mice" Molecules 18, no. 9: 11067-11085. https://doi.org/10.3390/molecules180911067




