First Chemical Constituents from Cordia exaltata Lam and Antimicrobial Activity of Two Neolignans
Abstract
:1. Introduction
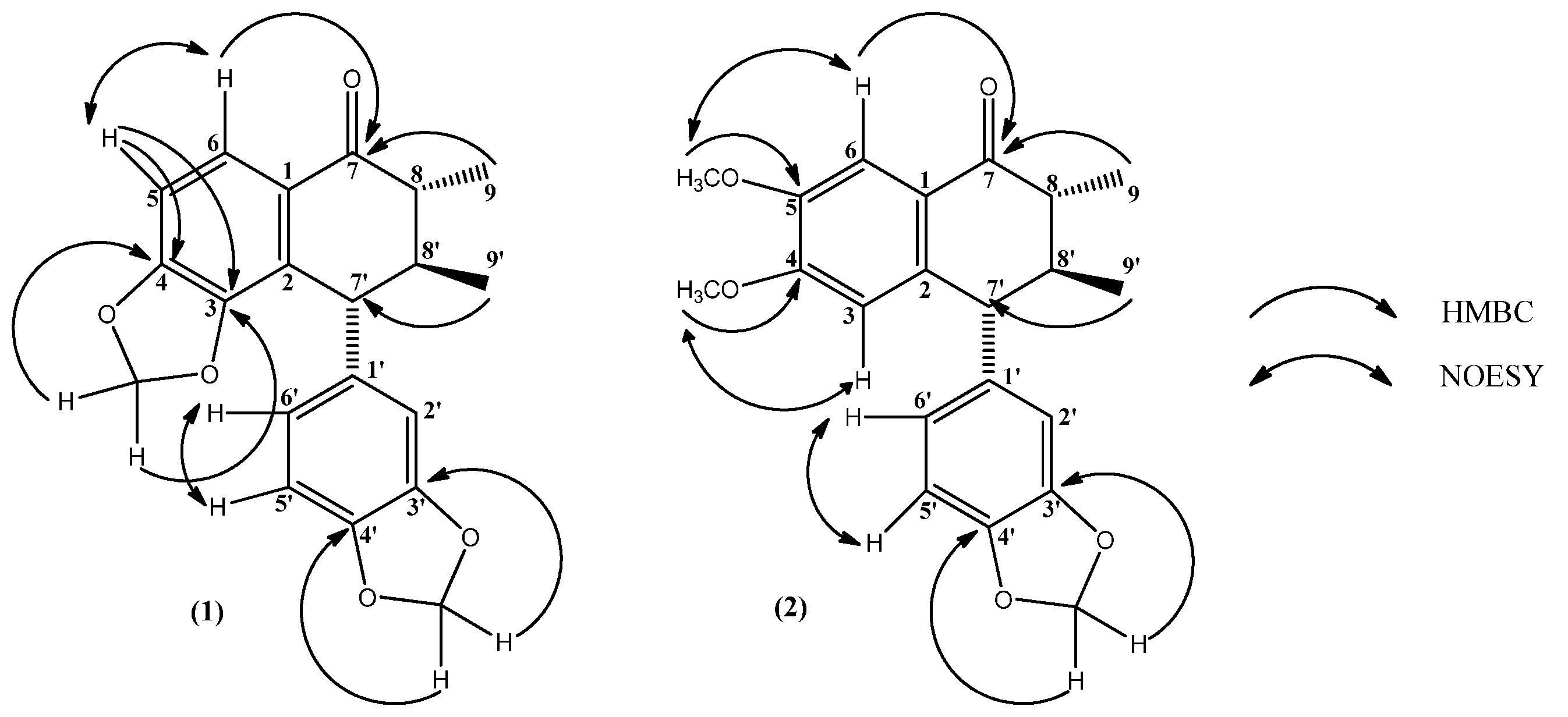
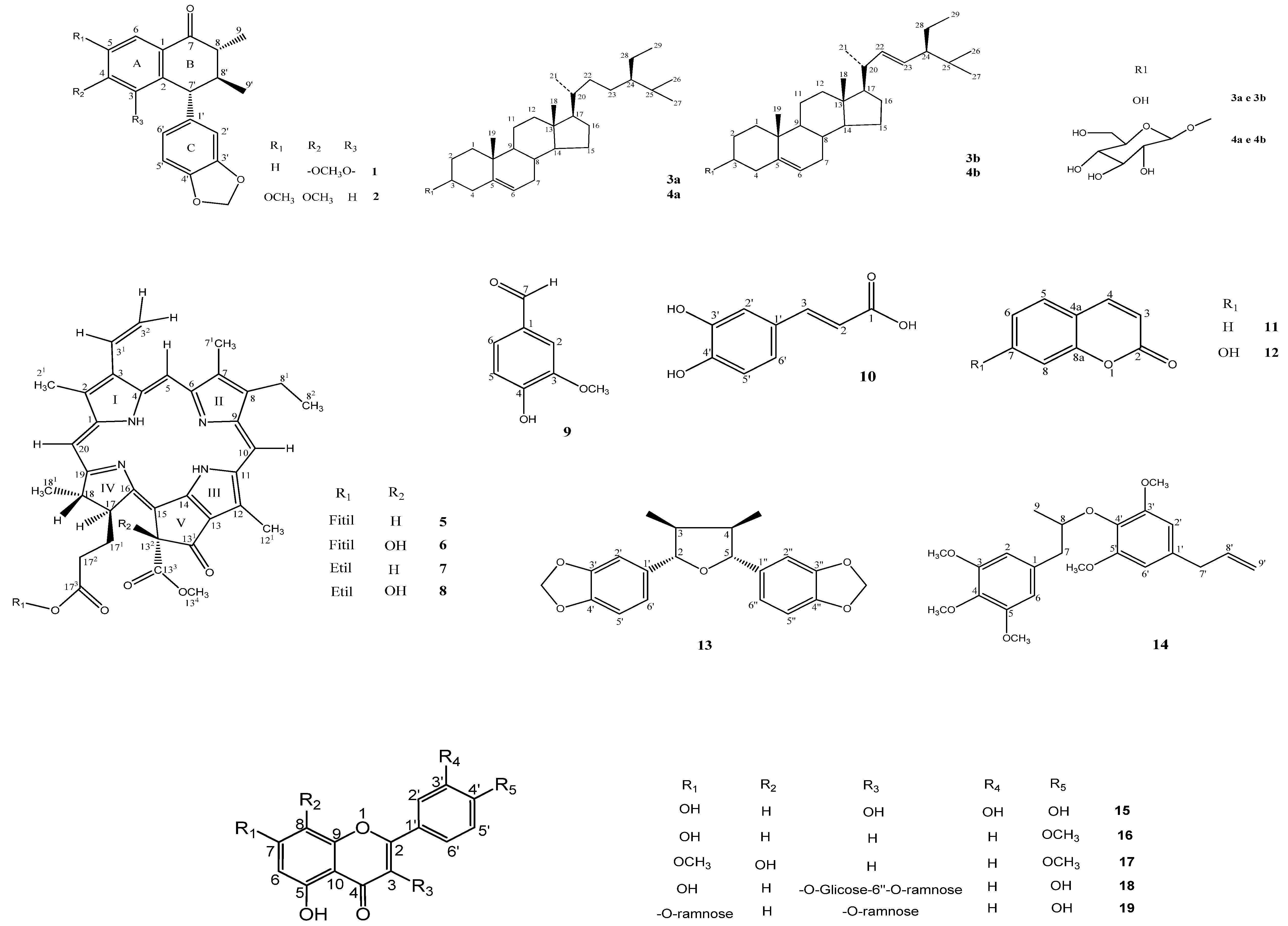
2. Results and Discussion
| Microorganisms | (1) (µg/mL) | (2) (µg/mL) | Controls | ||
|---|---|---|---|---|---|
| Tween 80 | Microrganisms | Antimicrobyal | |||
| S. aureus ATCC-6538 | 150 | >300 | + | + | - |
| S. aureus ATCC-25923 | 150 | >300 | + | + | + |
| S. epidermidis ATCC-12228 | 150 | >300 | + | + | - |
| B. subtilis ATCC-6633 | 150 | >300 | + | + | - |
| P. aeruginosa ATCC-25853 | 150 | 300 | + | + | + |
| P. aeruginosa ATCC-9027 | 150 | 300 | + | + | + |
| E. coli (clássica C) | 150 | 300 | + | + | - |
| E. coli ATCC-18739 | 150 | 300 | + | + | + |
| E. coli ATCC-8733 | 150 | >300 | + | + | + |
| S. flexineri LM-412 | 150 | 150 | + | + | - |
| C. albicans ATCC-90028 | 150 | 150 | + | + | - |
| C. albicans ATCC-76615 | 150 | 150 | + | + | - |
| C. albicans LM-142 V | 150 | 75 | + | + | - |
| C. albicans ICB-12 | 150 | 300 | + | + | - |
| C. tropicalis ATCC-13803 | >300 | 300 | + | + | + |
| C. tropicalis LM-028 | >300 | >300 | + | + | - |
| C. krusei ATCC-6258 | 150 | 75 | + | + | + |
| C. krusei LM-12 | 300 | 300 | + | + | - |
| C. guilliermondii LM-2101 | 75 | >300 | + | + | - |
| C. guilliermondii LM-011 | 75 | >300 | + | + | + |
3. Experimental
3.1. General
3.2. Collection, Extraction and Isolation
3.3. Spectral Data
3.4. Antimicrobial Activity Experiments
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Costa, J.F.O.; David, J.P.L.; David, J.M.; Giulietti, A.M.; Queiroz, L.P.; Santos, R.R.; Soares, M.B. Immunomodulatory activity of extracts from Cordia. superba Cham. and Cordia. rufescens A. DC. (Boraginaceae), Plant species native from Brazilian Semi-arid. Rev. Bras. Farmacogn. 2008, 18, 11–15. [Google Scholar]
- Arrebola, M.R.B.; Peterlin, M.F.; Bastos, D.H.M.; Rodrigues, R.F. De O.; Carvalho, P. De O. Estudo dos componentes lipídicos das sementes de três espécies do gênero Cordia. L. (Boraginaceae). Rev. Bras. Farmacogn. 2004, 14, 57–65. [Google Scholar] [CrossRef]
- Stevens, F. Angiosperm Phylogeny Website. Available online: http://www.mobot.mobot.org (accessed on 13 April 2013).
- Saito, M.L.; De Oliveira, F. Physical and chemical characteristics of a fuid extract of Cordia. ecalyculata Vell–Boraginaceae. Rev. Bras. Farmacogn. 1986, 1, 3–11. [Google Scholar] [CrossRef]
- Agra, M.F. Plantas. da medicina popular dos Cariris. Velhos, Paraíba-Brasil, 1st ed.; Editora União: João Pessoa, Brasil, 1996. [Google Scholar]
- Hayashi, K.; Hayashi, T.; Morita, N.; Niwayama, S. Antiviral activity of an extract of Cordia. salicifolia on Herpes simplex virus type I. Planta Med. 1990, 56, 439–443. [Google Scholar] [CrossRef]
- Rapisarda, A.; Barbeira, R.; De Pasquale, A.; Ficarra, P.; Ficarra, R.; Tommasini, S.; Calabrò, M.L.; Ragusa, S. Cordia francisci, C. martinicencis, C. myxa, C. serratifolia, and C. ulmifolia leaves as new sources of rutin: Analgesic and anti-inflammatory activity. Planta Med. S1. 1992, 58, 643–644. [Google Scholar]
- Ficarra, R.; Ficarra, P.; Tommasini, S.; Calabro, M.L.; Ragusa, S.; Barbera, R.; Rapisarda, A. Leaf extracts of some Cordia species: analgesic and anti-inflamatory activities as well as their chromatographic analysis. Farmaco 1995, 50, 245–256. [Google Scholar]
- Feng, P.C.; Haynes, L.J.; Magnus, K.E.; Plimmer, J.R.; Sherrat, H.S.A. Pharmacological screening of some west Indian medicinal plants. J. Pharmacol. 1962, 14, 556–561. [Google Scholar] [CrossRef]
- Ioset, J.R.; Martson, A.; Grupta, M.P.; Hostettmann, K. Antifungal and larvicidal compounds from the root bark of Cordia. alliadora. J. Nat. Prod. 2000, 63, 424–426. [Google Scholar]
- Marton, A.; Zagorski, M.G.; Hostettmann, K. Antifungal polyphenols from Cordia. goetzeigurke. Helv. Chim. Acta 1988, 71, 1210–1219. [Google Scholar] [CrossRef]
- Da Silva-Filho, A.A.; Lima, A.R.C.; Nascimento, S.C.; Silva, E.C.; Andrade, M.S; Lins, S.; Bieber, L.W. Biological activity of cordia quinones A and B, Isolated from Cordia. corymbosa. Fitoterapia 1993, 64, 78–80. [Google Scholar]
- Silverstein, R.M.; Webster, F.X. Identificação. Espectrométrica de Compostos Orgânicos, 7ª Edição ed; LTC: Rio de Janeiro, Brazil, 2000. [Google Scholar]
- Lee, T.H.; Yeh, M.H.; Chang, C.I.; Lee, C.K.; Shao, Y.Y.; Kuo, Y.H. New lignans from the Heartwood of Cunnighamialanceolata. Biosci. Biotechnol. Biochem. 2008, 71, 2075–2078. [Google Scholar]
- Kuo, Y.H.; Chen, C.H.; Chiang, Y.M. Three novel and one new lignan, Chamaecypanones A, B, obtulignolide and isoobanone from the heartwood of Chamaecyparisobtusa. var. formosana. Tetrahedron Lett. 2001, 42, 6731–6735. [Google Scholar] [CrossRef]
- Lopes, N.P.; Blumenthal, E.E.A.; Cavalheiro, A.J.; Kato, M.J.; Yoshida, M. Lignans, γ-Lactones and propiophenones of Virolasurinamensis. Phvtochemistry 1996, 43, 1089–1092. [Google Scholar]
- França, V.C. Estudo Fitoquímico de duas espécies de Aristolochiaceae: A. birostris Duchtr. e A. Papillaris. Master’s Thesis, Universidade Federal da Paraíba, João Pessoa, Brazil, 2003. [Google Scholar]
- Kojima, H.; Sato, N.; Hatano, A.; Ogura, H. Sterol glucosides from Prunellavulgaris. Phytochemistry 1990, 29, 2351–2355. [Google Scholar] [CrossRef]
- Matsuo, A.; Ono, K.; Hamasaki, K.; Nozaki, H. Phaeophytins from a cell suspension culture of the liverwort Plagiochilaovalifolia. Phytochemistry 1996, 42, 427–430. [Google Scholar]
- Jerz, G. Structural Chracterization of 132-hydroxy-(132-S)-phoephytin-A from leaves and stems of Amaranthus tricolor isolated by high-speed countercurrent chromatography. Innov. Food Sci. Emerg. Technol. 2007, 8, 413–418. [Google Scholar] [CrossRef]
- Chaves, O.S.; Gomes, R.A.; Tomaz, A.C.A.; Fernandes, M.G.; Junior, L.G.M.; Agra, M.F.; Braga, V.A.; Souza, M.F.V. Secondary metabolites from Sida. rhombifolia L. (Malvaceae) and the vasorelaxant activity of cryptolepinone. Molecules 2013, 18, 2769–2777. [Google Scholar] [CrossRef]
- Silva, D.A.; Silva, T.M.S.; Lins, A.C.S.; Costa, D.A.; Cavalcante, J.M.S.; Matias, W.N.; Souza, M.F.V. Constituintes químicos e atividade antioxidante de Sida. galheirensis Ulbr. (Malvaceae). Quim. Nova 2006, 29, 1250–1254. [Google Scholar] [CrossRef]
- Cavalcante, J.M.S.; Nogueira, T.B.S.S.; Tomaz, A.C.A.; Silva, D.A.; Agra, M.F.E.; Souza, M.F.V.; Carvalho, P.R.C.; Ramos, S.R.; Nascimento, S.C.; Gonçalves-Silva, T. Steroidal and Phenolic Compounds from Sidastrum. paniculatum(L.) Fryxell and Evaluation of Cytotoxic and Anti-inflammatory Activities. Quim. Nova 2010, 33, 846–849. [Google Scholar] [CrossRef]
- Queiroga, M.A. Investigação fitoquímica de Tillandsia recurvata L (Bromeliaceae). Master’s Thesis, Programa de Pós-Graduação em Produtos Naturais e Sintéticos Bioativos Universidade Federal da Paraíba, João Pessoa, Brasil, 2001. [Google Scholar]
- Chang, C.; Floss, H.G.; Steck, W. Carbon-13 Magnetic Resonance Spectroscopy of Coumarins. Carbon- 13-Proton Long-Range Couplings. J. Org. Chem. 1977, 42, 1337–1340. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Lima, R.A.; Monache, F.D. 13C nuclear magnetic resonance spectroscopy of 6- and 7-substituted coumarins. Correlation with Hammett constants. J. Chem. Soc., Perkin Trans. 2 1979, 4, 435–437. [Google Scholar] [CrossRef]
- Gomes, R.A.; Ramirez, R.R.A.; Maciel, J.K.S.; Agra, M.F.; Souza, M.F.V. Phenolic compounds from Sidastrum. micranthum (a. st.-hil.) fryxell and evaluation of acacetin and 7,4'-di-o-methylisoscutellarein as motulator of bacterial drug resistence. Quim. Nova 2011, 34, 1385–1388. [Google Scholar] [CrossRef]
- Horie, T.; Ohtsuru, Y.; Shibata, K.; Yamashiata, K.; Tsukayama, M.; Kawamura, Y. 13C-NMR Spectral Assignment of the A-ring of Polyoxygenated Flavones. Phytochemistry 1998, 47, 865–874. [Google Scholar] [CrossRef]
- Musayeib, N.A.; Perveen, S., Fátima; Nasir, M.; Hussain, A. Antioxidant, Anti-Glycation and Anti-inflammatory activities of phenolic constituents from Cordiasinensis. Molecules 2011, 16, 10214–10226. [Google Scholar] [CrossRef]
- Silva, D.A.; Costa, D.A.; Silva, D.F.; Agra, M.F.; Medeiros, I.A.; Braz Filho, R.; Souza, M.F.V. Flavonoides glicosilados de Herissantia. tiubae (K. Schum) Brizicky (Malvaceae) e testes farmacológicos preliminares do canferol 3,7-di-O-a-l-ramnopiranosídeo. Rev. Bras. Farmacogn. 2005, 15, 23–29. [Google Scholar] [CrossRef]
- Mitscher, L.A.; Lev, R.P.; Wu, N.; Beal, J.L.; Whitw, R. Antimicrobial agents from higher plants In: Introduction, Rational and methodology. Lloydia 1972, 35, 157–166. [Google Scholar]
- Alligianais, N.; Kalpoutzakis, E.; Mitaku, S.; Chinou, I.B. Composition and antimicrobial activity of the essential oil of two Origanum species. J. Agric. Food. Chem. 2001, 49, 4168–4179. [Google Scholar]
- Sartoratto, A.; Machado, A.L.M.; Delarmelina, C.; Figueira, G.M.; Duarte, M.C.T.; Rehder, V.L.G. Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Braz. J. Microbiol. 2004, 35, 275–280. [Google Scholar]
- Houghton, P.; Fang, R.; Techatanawat, I.; Steveton, G.; Hilands, P.J.; Lee, C.C. The sulphordamine (SRB) assay and other approaches to testing plant extracts and derived compounds for activies related to reputed anticancer activity. Methods 2007, 42, 377–387. [Google Scholar]
- Nunes, P.X.; Mesquita, R.F.; Silva, D.A.; Lira, P.L.; Costa, V.C.O.; Silva, V.B.; Xavier, A.L.; Diniz, M.F.F.M; Agra, M.F. Chemical constituents, Evaluation of the cytotoxic and antioxidant activities of Mimosa paraibana Barneby (Mimosaceae). Rev. Bras. Farmacogn. 2008, 18, 718–723. [Google Scholar]
- Costa, D.A.; Matias, W.N.; Lima, I.O.; Xavier, A.L.; Costa, V.B.M.; Diniz, M.F.F.M.; Agra, M.F.; Batista, L.M.; Souza, M.F.V. First secondary metabolites from Herissantia. crispa L (Brizicky) and the toxicity activity against Artemia. salina Leach. Quim. Nova 2009, 32, 48–50. [Google Scholar] [CrossRef]
- Bauer, A.W.M.M.; Kirby, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Amer. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar]
- Cleeland, R.; Squires, E. Evaluation of new antimicrobials “in vitro” and experimental animal infection. In Antibiotics in Laboratory Medicine; Willians. & Wikins: Baltimore, MD, USA, 1991. [Google Scholar]
- Hadacek, F.; Greger, H. Testing of antifungal natural products: Methodologies, Comparatibility of results and assay choice. Phytochem. Anal. 2000, 11, 137–147. [Google Scholar] [CrossRef]
- Koneman, E.W.; Allen, S.D.; Janda, W.M.; Schreckenberger, P.C.; winn Jr, W.C. Diagnóstco Microbiológico: Texto e Atlas Colorido, 5ª Edição ed; MEDSI-Editora Médica Científica Ltda: São Paulo, Brasil, 2001. [Google Scholar]
- Grabe, D.F. Manual de Teste. de Tetrazólio; Agiplan: Brasília, Brazil, 1976. [Google Scholar]
- Deswal, D.P.; Chand, U. Standardization of the tetrazolium test for viability estimation in ricebean (Vignaumbellata (Thumb) Ohwi&Ohashi) seeds. Seed Sci. Technol. 1997, 25, 409–417. [Google Scholar]
- Duarte, M.C.T.; Figueira, G.M.; Sartoratto, A.; Rehder, V.L.G.; Delarmelina, C. Anti-Candida activity of Brazilian medicinal plants. J. Ethnopharmacol. 2005, 97, 305–311. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–14 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bezerra de Sá de Sousa Nogueira, T.; Bezerra de Sá de Sousa Nogueira, R.; Antas e Silva, D.; Tavares, J.F.; De Oliveira Lima, E.; De Oliveira Pereira, F.; De Souza Fernandes, M.M.M.; De Medeiros, F.A.; Do Socorro Ferreira Rodrigues Sarquis, R.D.S.F.R.; Filho, R.B.; et al. First Chemical Constituents from Cordia exaltata Lam and Antimicrobial Activity of Two Neolignans. Molecules 2013, 18, 11086-11099. https://doi.org/10.3390/molecules180911086
Bezerra de Sá de Sousa Nogueira T, Bezerra de Sá de Sousa Nogueira R, Antas e Silva D, Tavares JF, De Oliveira Lima E, De Oliveira Pereira F, De Souza Fernandes MMM, De Medeiros FA, Do Socorro Ferreira Rodrigues Sarquis RDSFR, Filho RB, et al. First Chemical Constituents from Cordia exaltata Lam and Antimicrobial Activity of Two Neolignans. Molecules. 2013; 18(9):11086-11099. https://doi.org/10.3390/molecules180911086
Chicago/Turabian StyleBezerra de Sá de Sousa Nogueira, Tiago, Raquel Bezerra de Sá de Sousa Nogueira, Davi Antas e Silva, Josean Fechine Tavares, Edeltrudes De Oliveira Lima, Fillipe De Oliveira Pereira, Milen Maria Magalhães De Souza Fernandes, Fernando Antônio De Medeiros, Rosangela Do Socorro Ferreira Rodrigues Do Socorro Ferreira Rodrigues Sarquis, Raimundo Braz Filho, and et al. 2013. "First Chemical Constituents from Cordia exaltata Lam and Antimicrobial Activity of Two Neolignans" Molecules 18, no. 9: 11086-11099. https://doi.org/10.3390/molecules180911086




