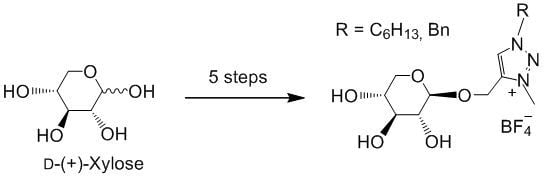
Click Reactions as a Key Step for an Efficient and Selective Synthesis of D-Xylose-Based ILs
Abstract
:1. Introduction
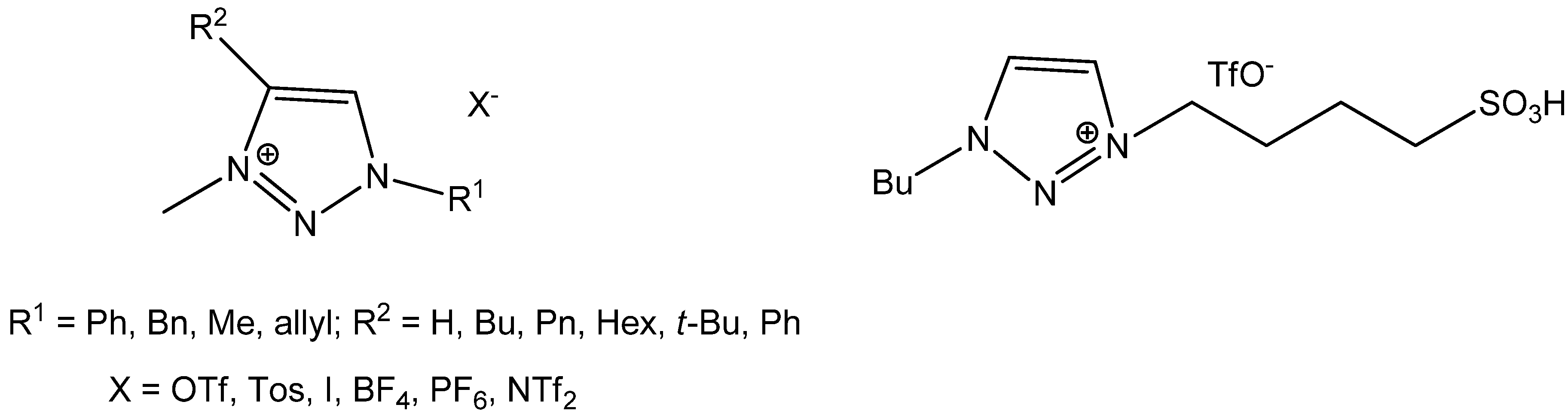
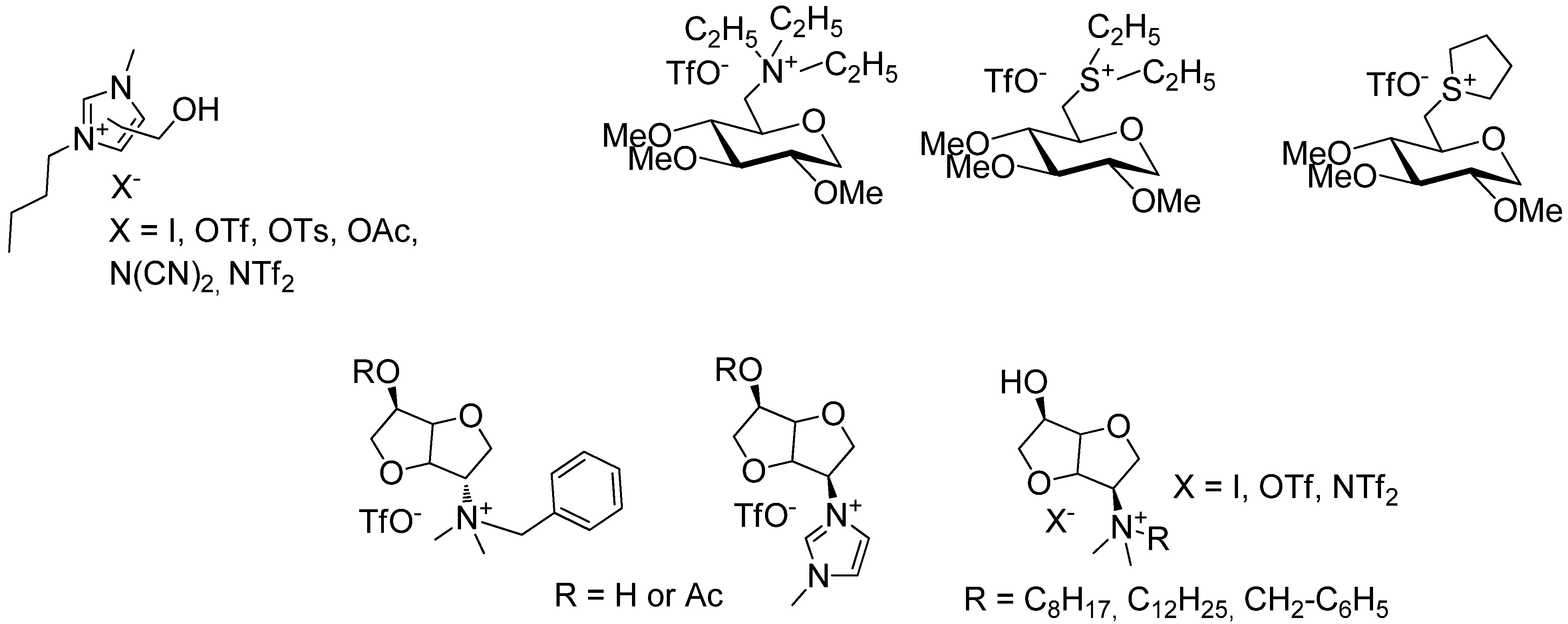
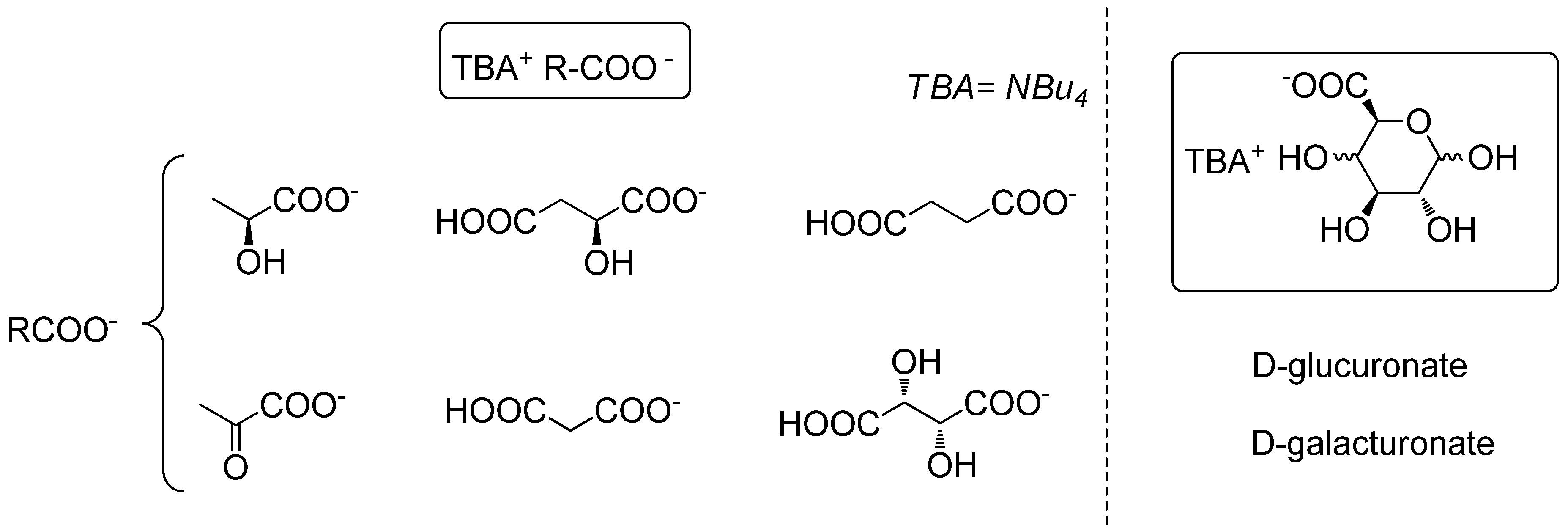
2. Results and Discussion
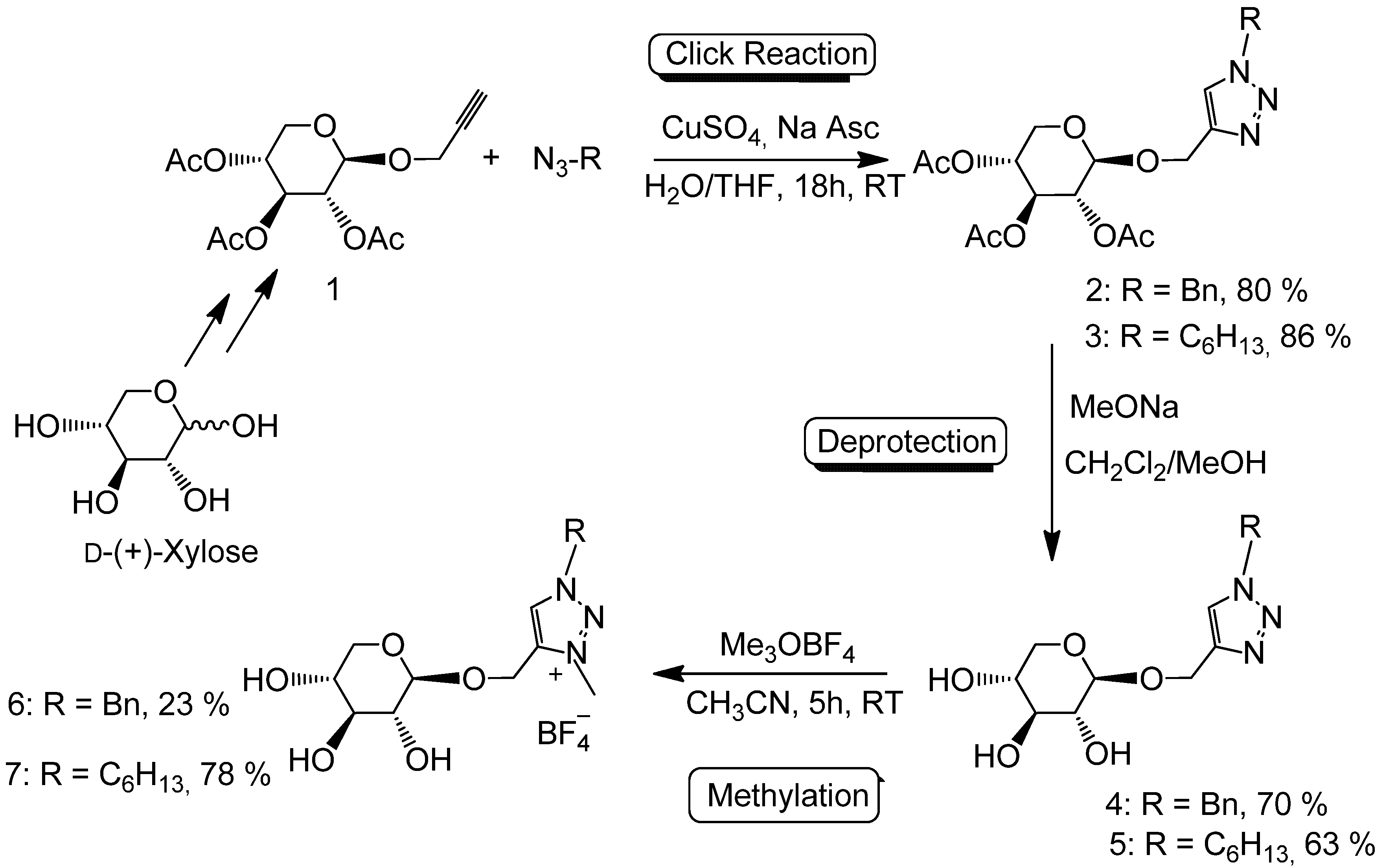
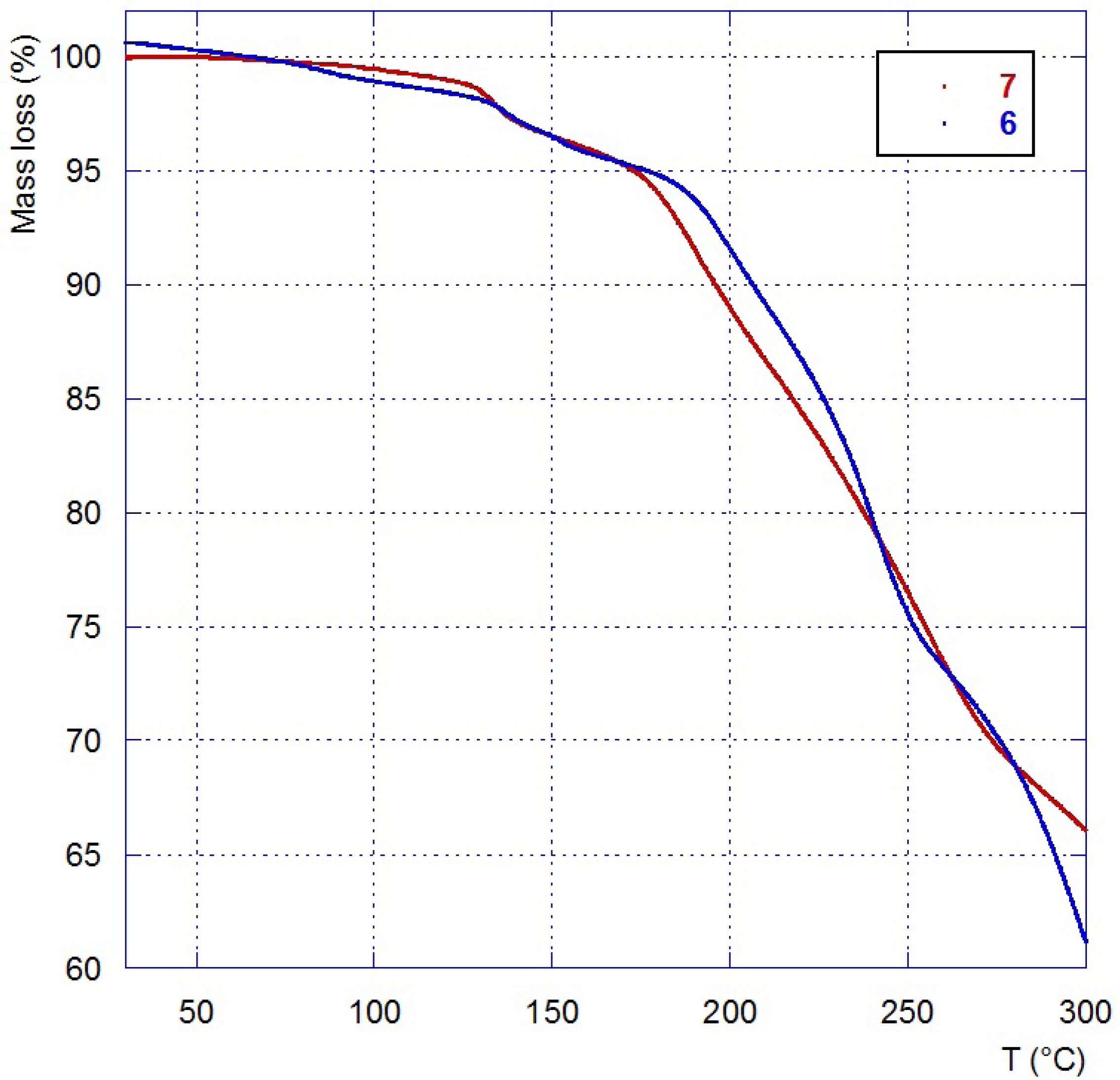
| IL | Tg (°C) a | Tdec (°C) b |
|---|---|---|
 | 4 | 150 |
| 6 | ||
 | 2.7 | 120 |
| 7 |
3. Experimental
3.1. General Procedures
3.2. Synthetic Procedures
3.2.1. Preparation of 1-((1-Benzyl-1,2,3-triazol-4-yl)methoxy)2,3,4-tri-O-acetyl-β-d-xylopyranoside (2)

3.2.2. Preparation of 1-((1-Hexyl-1,2,3-triazol-4-yl)methoxy)2,3,4-tri-O-acetyl-β-d-xylopyranoside (3)
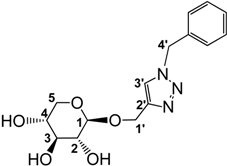
3.2.3. Preparation of 1-((1-Benzyl-1,2,3-triazol-4-yl)methoxy)β-d-xylopyranoside (4)
3.2.4. Preparation of 1-((1-Hexyl-1,2,3-triazol-4-yl)methoxy)β-d-xylopyranoside (5)

3.2.5. Preparation of 1-((1-Benzyl-3-methyl-1,2,3-triazol-4-yl)methoxy)β-d-xylopyranoside tetrafluoroborate (6)

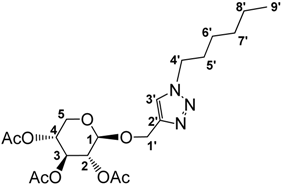
3.2.6. Preparation of 1-((1-Hexyl-3-methyl-1,2,3-triazol-4-yl)methoxy)β-d-xylopyranoside tetrafluoroborate (7)

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis; Wiley-VCH-Verlag GmbH & Co.: Weinheim, Germany, 2002. [Google Scholar]
- Earle, M.J.; Esperança, J.M.S.S.; Gilea, M.A.; Lopes, J.N.C.; Rebelo, L.P.N.; Magee, J.W.; Seddon, K.R.; Widegren, J.A. The distillation and volatility of ionic liquids. Nature 2006, 439, 831–834. [Google Scholar] [CrossRef]
- Couling, D.J.; Bernot, R.J.; Docherty, K.M.; Dixon, J.K.; Maginn, E.J. Assessing the factors responsible for ionic liquid toxicity to aquatic organisms via quantitative structure-property relationship modeling. Green Chem. 2006, 8, 82–90. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Magna, L.; Morvan, D. Ionic liquids and catalysis: Recent progress from knowledge to applications. Appl. Catal. A 2010, 373, 1–56. [Google Scholar] [CrossRef]
- Procuranti, B.; Myles, L.; Gathergood, N.; Connon, S.J. Pyridinium ion catalysis of carbonyl protection reactions. Synthesis 2009, 23, 4082–4086. [Google Scholar]
- Myles, L.; Gore, R.; Spulak, M.; Gathergood, N.; Connon, S.J. Highly recyclable, imidazolium derived ionic liquids of low antimicrobial and antifungal toxicity: A new strategy for acid catalysis. Green Chem. 2010, 12, 1157–1162. [Google Scholar] [CrossRef]
- Van Rantwijk, F.; Sheldon, R.A. Biocatalysis in ionic liquids. Chem. Rev. 2007, 107, 2757–2785. [Google Scholar] [CrossRef]
- Plaquevent, J.-C.; Levillain, J.; Guillen, F.; Malhiac, C.; Gaumont, A.-C. Ionic liquids: New targets and media for α-amino acid and peptide chemistry. Chem. Rev. 2008, 108, 5035–5060. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef]
- Wei, D.; Ivaska, A. Applications of ionic liquids in electrochemical sensors. Anal. Chim. Acta 2008, 607, 126–135. [Google Scholar] [CrossRef]
- Buzzeo, M.C.; Evans, R.G.; Compton, R.G. Non-haloaluminate room-temperature ionic liquids in electrochemistry—A review. ChemPhysChem 2004, 5, 1106–1120. [Google Scholar] [CrossRef]
- Galinski, M.; Lewandowski, A.; Stepniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Electrochemical Aspects of Ionic Liquids, 2nd ed.; Ohno, H. (Ed.) John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011.
- sterholm, A.; Damlin, P.; Kvarnström, C.; Ivaskaa, A. Studying electronic transport in polyazulene-ionic liquid systems using infrared vibrational spectroscopy. Phys. Chem. Chem. Phys. 2011, 13, 11254–11263. [Google Scholar] [CrossRef]
- Liu, J.-F.; Jiang, G.-B.; Jönsson, J.A. Application of ionic liquids in analytical chemistry. TrAC Trends Anal. Chem. 2005, 24, 20–27. [Google Scholar] [CrossRef]
- Baker, G.A.; Baker, S.N.; Pandey, S.; Bright, F.V. An analytical view of ionic liquids. Analyst 2005, 130, 800–808. [Google Scholar] [CrossRef]
- Pandey, S. Analytical applications of room-temperature ionic liquids: A review of recent efforts. Anal. Chim. Acta 2006, 556, 38–45. [Google Scholar] [CrossRef]
- Soukup-Hein, R.J.; Warnke, M.M.; Armstrong, D.W. Ionic liquids in analytical chemistry. Annu. Rev. Anal. Chem. 2009, 2, 145–168. [Google Scholar] [CrossRef]
- Sun, P.; Armstrong, D.W. Ionic liquids in analytical chemistry. Anal. Chim. Acta 2010, 661, 1–16. [Google Scholar] [CrossRef]
- Baltus, R.E.; Counce, R.M.; Culbertson, B.H.; Luo, H.; DePaoli, D.W.; Dai, S.; Duckworth, D.C. Examination of the potential of ionic liquids for gas separations. Sep. Sci. Technol. 2005, 40, 525–541. [Google Scholar] [CrossRef]
- Han, X.; Armstrong, D.W. Ionic liquids in separations. Acc. Chem. Res. 2007, 40, 1079–1086. [Google Scholar] [CrossRef]
- Arce, A.; Earle, M.J.; Katdare, S.P.; Rodriguez, H.; Seddon, K.R. Application of mutually immiscible ionic liquids to the separation of aromatic and aliphatic hydrocarbons by liquid extraction: A preliminary approach. Phys. Chem. Chem. Phys. 2008, 10, 2538–2542. [Google Scholar] [CrossRef]
- Luis, P.; Garea, A.; Irabien, A. Ionic liquids and membranes. J. Membr. Sci. 2009, 330, 80–89. [Google Scholar] [CrossRef]
- Gorri, D.; Ruiz, A.; Ortiz, A.; Ortiz, I. The use of ionic liquids as efficient extraction medium in the reactive separation of cycloolefines from cyclohexane. Chem. Eng. J. 2010, 154, 241–245. [Google Scholar] [CrossRef]
- Meindersma, G.W.; Hansmeier, A.R.; de Haan, A.B. Ionic liquids for aromatics extraction. Present status and future outlook. Ind. Eng. Chem. Res. 2010, 49, 7530–7540. [Google Scholar] [CrossRef]
- Leskinen, T.; King, A.W.T.; Kilpeläinen, I.; Argyropoulos, D.S. Fractionation of lignocellulosic materials with ionic liquids. Effect of mechanical treatment. Ind. Eng. Chem. Res. 2011, 50, 12349–12357. [Google Scholar]
- Huisgen, R. 1,3-Dipolar cycloaddition. Proc. Chem. Soc. 1961, 357–396. [Google Scholar]
- Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V.V.; Noodleman, L.; Sharpless, K.B.; Fokin, V.V. Copper(I)-catalyzed synthesis of azoles. DFT study predicts unprecedented reactivity and intermediates. J. Am. Chem. Soc. 2005, 127, 210–216. [Google Scholar]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar]
- Zekarias Yacob, Z.; Liebscher, J. 1,2,3-Triazolium Salts as a Versatile New Class of Ionic Liquids. “Ionic Liquids—Classes and Properties”; Handy, S.T., Ed.; InTech: Rijeka, Croatia, Chapter 1; 2011; pp. 1–22. [Google Scholar]
- Jeong, Y.-K.; Kim, D.-Y.; Choi, Y.-S.; Ryu, J.-S. Intramolecular hydroalkoxylation in Brønsted acidic ionic liquids and its application to the synthesis of (±)-centrolobine. Org. Biomol. Chem. 2011, 9, 374–378. [Google Scholar] [CrossRef]
- Zhao, F.Q.; Xue, L.; Xing, X.L.; Hu, R.Z.; Zhou, Z.M.; Gao, H.X.; Yi, J.H.; Xu, S.Y.; Pei, Q. Thermochemical properties and thermokinetic behavior of energetic triazole ionic salts. Sci. China Chem. 2011, 54, 461–474. [Google Scholar] [CrossRef]
- Fletcher, J.T.; Keeney, M.E.; Walz, S.E. 1-Allyl- and 1-Benzyl-3-methyl-1,2,3-triazolium salts via tandem click transformations. Synthesis 2010, 26, 3339–3345. [Google Scholar] [CrossRef]
- Jeong, Y.; Ryu, J.-S. Synthesis of 1,3-Dialkyl-1,2,3-triazolium ionic liquids and their applications to the baylis-hillman reaction. J. Org. Chem. 2010, 75, 4183–4191. [Google Scholar] [CrossRef]
- Hollingsworth, R.I.; Wang, G. Toward a carbohydrate-based chemistry: Progress in the development of general-purpose chiral synthons from carbohydrates. Chem. Rev. 2000, 100, 4267–4282. [Google Scholar] [CrossRef]
- Murugesan, S.; Karst, N.; Islam, T.; Wiencek, J.M.; Linhardt, R.J. Dialkyl imidazolium benzoates—Room temperature ionic liquids useful in the peracetylation and perbenzoylation of simple and sulfated saccharides. Synlett 2003, 9, 1283–1286. [Google Scholar]
- Chiappe, C.; Marra, A.; Mele, A. Synthesis and applications of ionic liquids derived from natural sugars. Top. Curr. Chem. 2010, 295, 177–195. [Google Scholar] [CrossRef]
- Forsyth, S.A.; MacFarlane, D.R.; Thomson, R.J.; von Itzstein, M. Rapid, clean, and mild O-acetylation of alcohols and carbohydrates in an ionic liquid. Chem. Commun. 2002, 7, 714–715. [Google Scholar]
- Rencurosi, A.; Lay, L.; Russo, G.; Caneva, E.; Poletti, L. Glycosylation with trichloroacetimidates in ionic liquids: Influence of the reaction medium on the stereochemical outcome. J. Org. Chem. 2005, 70, 7765–7768. [Google Scholar] [CrossRef]
- Murugesan, S.; Linhardt, R.J. Ionic liquids in carbohydrate chemistry—Current trends and future directions. Curr. Org. Synth. 2005, 2, 437–451. [Google Scholar] [CrossRef]
- Park, T.J.; Weiwer, M.; Yuan, X.J.; Baytas, S.N.; Munoz, E.M.; Murugesan, R.J. Linhardt. Glycosylation in room temperature ionic liquid using unprotected and unactivated donors. Carbohydr. Res. 2007, 342, 614–620. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Frade, R.F.M.; Afonso, C.A.M. Dissolution and transformation of carbohydrates in ionic liquids. Curr. Org. Synth. 2011, 8, 840–860. [Google Scholar]
- Galan, C.M.; Jones, R.A.; Tran, A.-T. Recent development of ionic liquids in oligosaccharide synthesis. The sweet side of ionic liquids. Carbohydr. Res. 2013, 375, 35–46. [Google Scholar] [CrossRef]
- Ogawa, C.; Kobayashi, S. Catalytic Asymmetric Synthesis, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2010; p. 1. [Google Scholar]
- Gaumont, A.-C.; Genisson, Y.; Guillen, F.; Plaquevent, J.-C. De Pasteur aux liquides ioniques chiraux. Petite histoire de l’induction asymétrique promue par le solvant. Actualité Chimique 2011, 348–349, 84–89. [Google Scholar]
- Gaumont, A.-C.; Genisson, Y.; Guillen, F.; Zgonnik, V.; Plaquevent, J.-C. Chiral ionic liquids for asymmetric reactions. In Catalytic Methods in Asymmetric Synthesis; Gruttadauria, M., Giaccalone, F., Eds.; John Wiley & Sons, Inc.: Palermo, Italy, 2011; pp. 323–344. [Google Scholar]
- Chen, X.; Ying, A. Ionic liquids: Applications and perspectives. In Ionic Liquids: Applications and Perspectives; Kokorin, A., Ed.; InTech: Rijeka, Croatia, 2011; pp. 305–330. [Google Scholar]
- Payagala, T.; Armstrong, D.W. Chiral ionic liquids: A compendium of syntheses and applications. Chirality 2012, 24, 17–53. [Google Scholar] [CrossRef]
- Ding, J.; Welton, T.; Armstrong, D.W. Chiral ionic liquids as stationary phases in gas chromatography. Anal. Chem. 2004, 76, 6819–6822. [Google Scholar] [CrossRef]
- Handy, S.T.; Okello, M.; Dickenson, G. Solvents from biorenewable sources: Ionic liquids based on fructose. Org. Lett. 2003, 5, 2513–2515. [Google Scholar] [CrossRef]
- Poletti, L.; Chiappe, C.; Lay, L.; Pieraccini, D.; Polito, L.; Russo, G. Glucose-derived ionic liquids: Exploring low-cost sources for novel chiral solvents. Green Chem. 2007, 9, 337–341. [Google Scholar] [CrossRef]
- Quadir, M.; Mathonneau, E. Cosmetic Composition Comprising Multiphasic Particles. U.S. Patent 20070231355, 4 October 2007. [Google Scholar]
- Pereira, M.; Manuela, A. Chiral ionic liquids from carbohydrates: Synthesis and properties. MiniRev. Org. Chem. 2012, 9, 243–260. [Google Scholar] [CrossRef]
- Truong, T.-K.-T.; van Buu, O.N.; Aupoix, A.; Pegot, B.; Vo-Thanh, G. Chiral ionic liquids derived from (−)-ephedrine and carbohydrates: Synthesis, properties and applications to asymmetric synthesis and catalysis. Curr. Org. Synth. 2012, 9, 53–64. [Google Scholar] [CrossRef]
- Kumar, V.; Pei, C.; Olsen, C.E.; Schäffer, S.J.C.; Parmar, V.S.; Malhotra, S.V. Novel carbohydrate-based chiral ammonium ionic liquids derived from isomannide. Tetrahedron Asymmetry 2008, 19, 664–671. [Google Scholar] [CrossRef]
- Nguyen Van Buu, O.; Aupoix, A.; Vo-Thanh, G. Synthesis of novel chiral imidazolium-based ionic liquids derived from isosorbide and their applications in asymmetric aza Diels–Alder reaction. Tetrahedron 2009, 65, 2260–2265. [Google Scholar] [CrossRef]
- Nguyen Van Buu, O.; Aupoix, A.; Doan Thi Hong, N.; Vo-Thanh, G. Chiral ionic liquids derived from isosorbide: Synthesis, properties and applications in asymmetric synthesis. New J. Chem. 2009, 33, 2060–2072. [Google Scholar] [CrossRef]
- Ferlin, N.; Courty, M.; Gatard, S.; Spulak, M.; Quilty, B.; Beadham, I.; Ghavre, M.; Gathergood, N.; Bouquillon, S. Biomass derived ionic liquids: Synthesis from natural organic acids, characterization, toxicity, biodegradation and use as solvents for catalytic hydrogenation processes. Tetrahedron 2013, 69, 6150–6161. [Google Scholar] [CrossRef]
- Estrine, B.; Bouquillon, S.; Hénin, F.; Muzart, J. Telomerization of butadiene with l-arabinose and d-xylose in DMF: Selective formation of their monooctadienyl glycosides. Eur. J. Org. Chem. 2004, 2914–2922. [Google Scholar]
- Estrine, B.; Bouquillon, S.; Hénin, F.; Muzart, J. Telomerization of butadiene with pentoses in water: Selective etherifications. Green Chem. 2005, 7, 219–223. [Google Scholar] [CrossRef]
- Hadad, C.; Damez, C.; Bouquillon, S.; Estrine, B.; Hénin, F.; Muzart, J.; Pezron, I.; Komunjer, L. Neutral pentosides surfactants issued from the butadiene telomerization with pentoses: Preparation and amphiphilic properties. Carbohydr. Res. 2006, 341, 1938–1944. [Google Scholar] [CrossRef]
- Damez, C.; Bouquillon, S.; Harakat, D.; Hénin, F.; Muzart, J.; Pezron, I.; Komunjer, L. Alkenyl and alkenoyl amphiphilic derivatives of d-xylose and their surfactant properties. Carbohydr. Res. 2007, 342, 154–162. [Google Scholar] [CrossRef]
- Deleu, M.; Damez, C.; Gatard, S.; Nott, K.; Paquot, M.; Bouquillon, S. Synthesis and physico-chemical characterization of bolaamphiphiles derived from alkenyl d-xylosides. New J. Chem. 2011, 35, 2258–2266. [Google Scholar] [CrossRef]
- Deleu, M.; Gatard, S.; Payen, E.; Lins, L.; Nott, K.; Flore, C.; Thomas, R.; Paquot, M.; Bouquillon, S. d-xylose-based bolaamphiphiles: Synthesis and influence of the spacer nature on their interfacial and membrane properties. C. R. Chim. 2012, 15, 68–74. [Google Scholar] [CrossRef]
- Hadad, C.; Majoral, J.P.; Muzart, J.; Caminade, A.M.; Bouquillon, S. First phosphorous d-xylose derived glycodendrimers. Tetrahedron Lett. 2009, 50, 1902–1905. [Google Scholar] [CrossRef]
- Camponovo, J.; Hadad, C.; Ruiz, J.; Cloutet, E.; Gatard, S.; Muzart, J.; Bouquillon, S.; Astruc, D. “Click” glycodendrimers containing 27, 81 and 243 modified xylopyranoside termini. J. Org. Chem. 2009, 74, 5071–5074. [Google Scholar] [CrossRef]
- Gatard, S.; Liang, L.; Salmon, L.; Ruiz, J.; Astruc, D.; Bouquillon, S. Water-soluble glycodendrimers: Synthesis and use in hydrogenation catalytic process. Tetrahedron Lett. 2011, 52, 1842–1846. [Google Scholar] [CrossRef]
- Hanelt, S.; Liebscher, J. A novel and versatile access to task-specific ionic liquids based on 1,2,3-Triazolium salts. Synlett 2008, 7, 1058–1060. [Google Scholar] [CrossRef]
- Shah, J.; Kahn, S.S.; Blumenthal, H.; Liebscher, J. 1,2,3-Triazolium-tagged prolines and their application in asymmetric aldol and Michael reactions. Synthesis 2009, 23, 3975–3982. [Google Scholar]
- Nakamura, T.; Ogata, K.; Fukuzawa, S.-I. Synthesis of dichlorobis(1,4-dimesityl-1H-1,2,3-triazol-5-ylidene)palladium [PdCl2(TMes)2] and its application to suzuki-miyaura coupling reaction. Chem. Lett. 2010, 39, 920–922. [Google Scholar] [CrossRef]
- Nulwala, H.B.; Tang, C.N.; Kail, B.W.; Damodaran, K.; Kaur, P.; Wickramanayake, S.; Shi, W.; Luebke, D.R. Probing the structure-property relationship of regioisomeric ionic liquids with click chemistry. Green Chem. 2011, 13, 3345–3349. [Google Scholar] [CrossRef]
- Aizpurua, J.M.; Sagartzazu-Aizpurua, M.; Azcune, I.; Miranda, J.I.; Monasterio, Z.; García-Lecina, E.; Fratila, R.M. “Click” synthesis of nonsymmetrical 4,4'-Bis(1,2,3-triazolium) salts. Synthesis 2011, 17, 2737–2742. [Google Scholar]
- Ohmatsu, K.O.; Kiyokawa, M.; Ooi, T. Chiral 1,2,3-Triazoliums as new cationic organic catalysts with anion-recognition ability: Application to asymmetric alkylation of oxindoles. J. Am. Chem. Soc. 2011, 133, 1307–1309. [Google Scholar] [CrossRef]
- Khan, S.S.; Shah, J.; Liebscher, J. Ionic-liquid tagged prolines as recyclable organocatalysts for enantioselective α-aminoxylations of carbonyl compounds. Tetrahedron 2011, 67, 1812–1820. [Google Scholar]
- Yoshida, Y.; Takizawa, S.; Sasai, H. Design and synthesis of spiro bis(1,2,3-triazolium) salts as chiral ionic liquids. Tetrahedron Asymmetry 2012, 23, 843–851. [Google Scholar] [CrossRef]
- Sanghi, S.; Willett, E.; Versek, C.; Tuominen, M.; Coughlin, E.B. Physicochemical properties of 1,2,3-triazolium ionic liquids. RSC Adv. 2012, 2, 848–853. [Google Scholar] [CrossRef]
- Mereyala, H.B.; Gurrala, S.R. A highly diastereoselective, practical synthesis of allyl, propargyl 2,3,4,6-tetra-O-acetyl-β-d-gluco- or β-d-galactopyranosides and allyl, propargyl heptaacetyl-β-d-lactosides. Carbohydr. Res. 1998, 307, 351–354. [Google Scholar] [CrossRef]
- Fischer, E. Ueber die verbindungen der zucker mit den alkoholen und ketonen. Ber. Dtsch. Chem. Ges. 1895, 28, 1145–1167. [Google Scholar] [CrossRef]
- Schulze, B.; Friebe, C.; Hager, M.D.; Günther, W.; Köhn, U.; Jahn, B.O.; Görls, H.; Schubert, U.S. Anion complexation by triazolium “ligands”: Mono- and bis-tridentate complexes of sulfate. Org. Lett. 2010, 12, 2710–2713. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 2–7 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ferlin, N.; Gatard, S.; Van Nhien, A.N.; Courty, M.; Bouquillon, S. Click Reactions as a Key Step for an Efficient and Selective Synthesis of D-Xylose-Based ILs. Molecules 2013, 18, 11512-11525. https://doi.org/10.3390/molecules180911512
Ferlin N, Gatard S, Van Nhien AN, Courty M, Bouquillon S. Click Reactions as a Key Step for an Efficient and Selective Synthesis of D-Xylose-Based ILs. Molecules. 2013; 18(9):11512-11525. https://doi.org/10.3390/molecules180911512
Chicago/Turabian StyleFerlin, Nadège, Sylvain Gatard, Albert Nguyen Van Nhien, Matthieu Courty, and Sandrine Bouquillon. 2013. "Click Reactions as a Key Step for an Efficient and Selective Synthesis of D-Xylose-Based ILs" Molecules 18, no. 9: 11512-11525. https://doi.org/10.3390/molecules180911512