Synthesis, Anticancer Activity and UPLC Analysis of the Stability of Some New Benzimidazole-4,7-dione Derivatives
Abstract
:1. Introduction

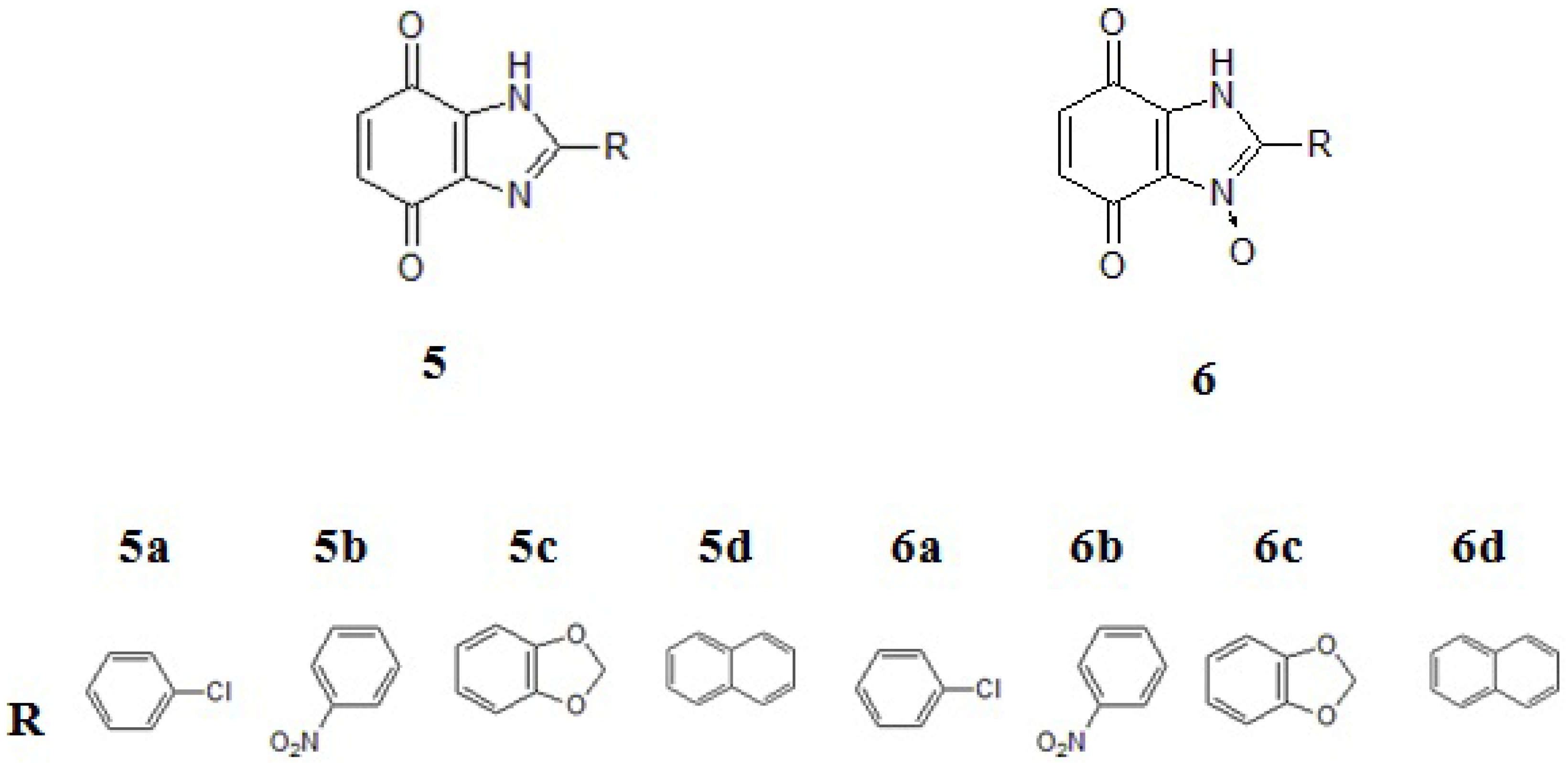
2. Results and Discussion
2.1. Chemistry

2.2. UPLC Analysis
2.2.1. Optimisation
| Concetration 10−1 mg/mL | Rtmean (min) | kmean | nmean |
|---|---|---|---|
| 5b | 4.3 | 1.24e^1 | 7.74e^3 |
| 6b | 3.9 | 1.20e^1 | 6.31e^3 |
2.2.2. Validation
2.2.3. Stability of Compounds in Different Environments
| Compound 5b | |
| Solvent | Recovery (%) |
| 0.9% NaCl | 77.0 |
| H2O | 100.0 |
| 0.2% DMSO | 83.0 |
| MeOH | 98.2 |
| Compound 6b | |
| 0.9% NaCl | 78.2 |
| H2O | 77.9 |
| 0.2% DMSO | 90.4 |
| MeOH | 76.1 |
| Compound 5b | |||
| Solvent | k (h−1) | t1/2 (h) | R2 |
| 0.9% NaCl | 0.0004 | 155 | 0.98 |
| H2O | a | a | a |
| 0.2% DMSO | 0.0002 | 215 | 0.93 |
| MeOH | a | a | a |
| Compound 6b | |||
| 0.9% NaCl | 0.0004 | 142 | 0.97 |
| H2O | 0.0004 | 146 | 0.86 |
| 0.2% DMSO | 0.00009 | 413 | 0.99 |
| MeOH | 0.0002 | 161 | 0.99 |
2.3. Biological Activities under Normoxia and Hypoxia Conditions
2.3.1. Cytotoxicity at Normoxia
| Compound | IC50 [μM] A549 | Differential cytotoxicity O/H | |
|---|---|---|---|
| Normoxia (O) | Hypoxia (H) | ||
| 5a | 30.2 ± 1.2 | 36.1 ± 1.2 | 0.83 |
| 5b | 100.0 ± 1.8 | 47.4 ± 1.1 | 2.13 |
| 5c | 80.9 ± 1.9 | 51.2±1.5 | 1.58 |
| 5d | 479.5 ± 3.6 | 232.4 ± 1.4 | 2.06 |
| 6a | 115.7 ± 1.9 | 35.0 ± 1.6 | 3.28 |
| 6b | 500.6 ± 1.2 | 116.0 ± 0.8 | 4.31 |
| 6c | 79.5 ± 1.9 | 44.0 ± 2.5 | 1.80 |
| 6d | 252 ± 2.5 | 96.8 ± 1.9 | 2.62 |
| Tirapazamine | 166.2 ± 1.6 | 30.0 ± 1.5 | 4.61 |
2.3.2. Cytotoxicity at Hypoxia
3. Experimental
3.1. Materials and Instrumentation
3.2. Methods
3.2.1. Chemistry
3.2.2. UPLC Analysis
3.2.2.1. Optimisation
3.2.2.2. Validation and Evaluation Procedures of the Compounds Stability in Different Media

3.2.3. Cytotoxicity
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Perry, M.D.J.; Carvalho, E.; Rosa, E.; Iley, J. Towards an efficient prodrug of the alkylating metabolite monomethyltriazene: Synthesis and stability of N-acylamino acid derivatives of triazenes. Eur. J. Med. Chem. 2009, 44, 1049–1056. [Google Scholar] [CrossRef]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Can. 2011, 1, 393–410. [Google Scholar] [CrossRef]
- Denny, W.A. The role of hypoxia-activated prodrugs in cancer therapy. Lancet Oncol. 2000, 1, 25–29. [Google Scholar] [CrossRef]
- Chung-Faye, G.; Palmer, D.; Anderson, D.; Clark, J.; Downes, M.; Baddeley, J.; Hussain, S.; Murray, P.I.; Searle, P.; Seymour, L.; et al. Virus-directed, enzyme prodrug therapy with nitroimidazole reductase: A phase I and pharmacokinetic study of its prodrug, CB1954. Clin. Cancer. Res. 2001, 7, 2662–2668. [Google Scholar]
- Tomasz, M. Mitomycin C: Small, fast and deadly (but very selective). Chem. Biol. 1995, 2, 575–579. [Google Scholar] [CrossRef]
- Brown, J.M. The hypoxic cell: A target for selective cancer therapy—Eighteenth Bruce F. Cain memorial award lecture. Cancer Res. 1999, 59, 5863–5870. [Google Scholar]
- Koch, C.J. Unusual oxygen concentration dependence of toxicity of SR-4233, a hypoxic cell toxin. Cancer Res. 1993, 53, 3992–3997. [Google Scholar]
- Siim, B.G.; Pruijn, F.B.; Sturman, J.R.; Hogg, A.; Hay, M.P.; Brown, J.M.; Wilson, W.R. Selective potentiation of the hypoxic cytotoxicity of Tirapazamine by its 1-N-oxide metabolite SR 4317. Cancer Res. 2004, 64, 736–742. [Google Scholar] [CrossRef]
- Siemann, W.; Hinchman, C.A. Potentiation of cisplatin activity by the bioreductive agent tirapazamine. Radiother. Oncol. 1998, 47, 215–220. [Google Scholar] [CrossRef]
- Ciesielska, E.; Szulawska, A.; Studzian, K.; Ochocki, J.; Malinowska, K.; Kik, K.; Szmigiero, L. Comparative studies on the mechanism of cytotoxic action of novel platinum II complexes with pyrazole ligands. J. Inorg. Biochem. 2006, 100, 1579–1585. [Google Scholar] [CrossRef]
- Kalinowska-Lis, U.; Szmigiero, L.; Studzian, K.; Ochocki, J. Cytotoxic activity and chemical reactivity of cis-platinum(II) and trans-palladium(II) complexes with diethyl (pyridinylmethyl)phosphates. Eur. J. Med. Chem. 2009, 44, 660–664. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Szmigiero, L.; Zyner, E.; Ochocki, J. Proapoptotic activity in vitro of two novel ruthenium(II) complexes with flavanone-based ligands that overcome cisplatin resistance in human bladder carcinoma cells. J. Inorg. Biochem. 2011, 105, 518–524. [Google Scholar] [CrossRef]
- Papadopoulou, M.V.; Bloomer, W.D. NLCQ-1 (NSC 709257): Exploiting hypoxia with a weak DNA-intercalating bioreductive drug. Clin. Cancer Res. 2003, 9, 5714–5720. [Google Scholar]
- Patterson, L.H.; McKeown, S.R. AQ4N: A new approach to hypoxia-activated cancer chemotherapy. Br. J. Cancer 2000, 83, 1589–1593. [Google Scholar] [CrossRef]
- Błaszczak-Świątkiewicz, K.; Mirowski, M.; Kaplińska, K.; Kruszyński, R.; Trzęsowska-Kruszyńska, A.; Mikiciuk-Olasik, E. New benzimidazole derivatives with potential cytotoxic activity—Study of their stability by RP-HPLC. Acta Biochim. Pol. 2012, 59, 279–288. [Google Scholar]
- Jin, S.; Kim, J.S.; Sim, S.P.; Liu, A.; Pilch, D.S.; Liu, L.F.; LaVoie, E.J. Heterocyclic bibenzimidazole derivatives as topoisomerase I inhibitors. Bioorg. Med. Chem. Lett. 2000, 10, 719–723. [Google Scholar] [CrossRef]
- Alpan, A.S.; Gunes, H.S.; Topcu, Z. 1H-Benzimidazole derivatives as mammalian DNA topoisomerase I inhibitors. Acta Biochim. Pol. 2007, 54, 561–565. [Google Scholar]
- Garuti, L.; Roberti, M.; Malagoli, M.; Rossi, T.; Castelli, M. Synthesis and antiproliferative activity of some benzimidazole-4,7-dione derivatives. Bioorg. Med. Chem. Lett. 2000, 10, 2193–2195. [Google Scholar] [CrossRef]
- Antonini, I.; Claudi, F.; Cristalli, G.; Franchetti, P.; Grifantini, M.; Martelli, S. Heterocyclic quinones with potential antitumor activity. Synthesis, antitumor activity of some benzimidazole-4,7-dione derivatives. J. Med. Chem. 1988, 31, 260–264. [Google Scholar] [CrossRef]
- Garuti, L.; Roberti, M.; Pizzirani, D.; Pession, A.; Leoncini, E.; Cenci, V.; Hrelia, S. Differential antiproliferative activity of new benzimidazole-4,7-diones. Il Farmaco 2004, 59, 663–668. [Google Scholar] [CrossRef]
- Boufatah, N.; Gellis, A.; Maldonado, J.; Vanelle, P. Efficient microwave-assisted synthesis of new sulfonylbenzimidazole-4,7-diones: Heterocyclic quinones with potential antitumor activity. Tetrahedron 2004, 60, 9131–9137. [Google Scholar] [CrossRef]
- Coban, G.; Zencir, S.; Zupko, I.; Rethy, B.; Gunes, H.S.; Topcu, Z. Synthesis and biological activity evaluation of 1H-benzimidazoles via mammalian DNA topoisomerase I and cytostaticity assays. Eur. J. Med. Chem. 2009, 44, 2280–2285. [Google Scholar] [CrossRef]
- Oksuzoglu, E.; Tekiner-Gulbas, B.; Amper, S. Some benzoxazoles and benzimidazoles as DNA topoisomerase I and II inhibitors. J. Enz. Inh. Med. Chem. 2008, 23, 37–42. [Google Scholar] [CrossRef]
- Omyła-Staszewska, J.; Deptała, A. Inhibitory topoizomerazy I—Unikalna grupa leków przeciwnowotworowych. Współ. Onko. 2003, 7, 45–53. [Google Scholar]
- Singh, M.; Tandon, V. Synthesis and biological activity of novel inhibitors of topoisomerase I: 2-Aryl-substituted 2-bis-1H-benzimidazoles. Eur. J. Med. Chem. 2011, 46, 659–669. [Google Scholar] [CrossRef]
- Wu, N.; Wu, X.; Agama, K.; Pommier, Y.; Du, J.; Li, D.; Gu, L.-Q.; Huang, Z.-S.; An, L.-K. A novel DNA topoisomerase I inhibitor with different mechanism from camptothecin induces G2/M phase cell cycle arrest to K562 cells. Biochemistry 2010, 49, 10131–10136. [Google Scholar] [CrossRef]
- Alper, S.; Arpaci, O.T.; Aki, E.S.; Yalcin, I. Some new bi- and ter-benzimidazole derivatives as topoisomerase Inhibitors. II Farmaco 2003, 58, 497–507. [Google Scholar] [CrossRef]
- Alpan, A.S.; Zencir, S.; Zupko, S.Z.; Coban, G.; Rethy, B.; Gunes, H.S.; Topcu, Z. Biological activity of bis-benzimidazole derivatives on DNA topoisomerase I and HeLa, MCF7 and A431 cells. J. Enzym. Inhib. Med. Chem. 2009, 24, 844–849. [Google Scholar] [CrossRef]
- Błaszczak-Świątkiewicz, K.; Mikiciuk-Olasik, E. Application of HPLC method for investigation of stability of new benzimidazole derivatives. J. Liq. Chrom. Rel. Tech. 2011, 34, 1901–1912. [Google Scholar] [CrossRef]
- Błaszczak-Świątkiewicz, K.; Olszewska, P.; Mikiciuk-Olasik, E. Biological evaluation of activity of new benzimidazole derivatives. Acta Biochim. Pol. 2013, 60, 427–433. [Google Scholar]
- Mikiciuk-Olasik, E.; Błaszczak-Świątkiewicz, K.; Żurek, E.; Krajewska, U.; Różalski, M.; Kruszyński, R.; Bartczak, T.J. New derivatives of quinazoline and 1,2-dihydroquinazoline N3 –oxide with expected antitumour acivity. Arch. Pharm. Pharm. Med. Chem. 2004, 5, 239–246. [Google Scholar]
- Vogel, A.I.; Furniss, B.S. Vogel’s Textbook of Practical Chemistry Including Qualitative Organic; The English Language Book Society Analysis: London, UK, 1978. [Google Scholar]
- Sample Availability: Samples of the compounds 5a–d and 6a–d are available from the authors.
© 2013 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Błaszczak-Świątkiewicz, K.; Almeida, D.C.; Perry, M.D.J.; Mikiciuk-Olasik, E. Synthesis, Anticancer Activity and UPLC Analysis of the Stability of Some New Benzimidazole-4,7-dione Derivatives. Molecules 2014, 19, 400-413. https://doi.org/10.3390/molecules19010400
Błaszczak-Świątkiewicz K, Almeida DC, Perry MDJ, Mikiciuk-Olasik E. Synthesis, Anticancer Activity and UPLC Analysis of the Stability of Some New Benzimidazole-4,7-dione Derivatives. Molecules. 2014; 19(1):400-413. https://doi.org/10.3390/molecules19010400
Chicago/Turabian StyleBłaszczak-Świątkiewicz, Katarzyna, Diogo Correia Almeida, Maria De Jesus Perry, and Elżbieta Mikiciuk-Olasik. 2014. "Synthesis, Anticancer Activity and UPLC Analysis of the Stability of Some New Benzimidazole-4,7-dione Derivatives" Molecules 19, no. 1: 400-413. https://doi.org/10.3390/molecules19010400
APA StyleBłaszczak-Świątkiewicz, K., Almeida, D. C., Perry, M. D. J., & Mikiciuk-Olasik, E. (2014). Synthesis, Anticancer Activity and UPLC Analysis of the Stability of Some New Benzimidazole-4,7-dione Derivatives. Molecules, 19(1), 400-413. https://doi.org/10.3390/molecules19010400




