Up-Regulation of Urotensin II and Its Receptor Contributes to Human Hepatocellular Carcinoma Growth via Activation of the PKC, ERK1/2, and p38 MAPK Signaling Pathways
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hematoxylin and Eosing Staining and Alpha-Fetoprotein Expression in the Liver

2.2. mRNA and Protein Expression of UII and UT in HCC
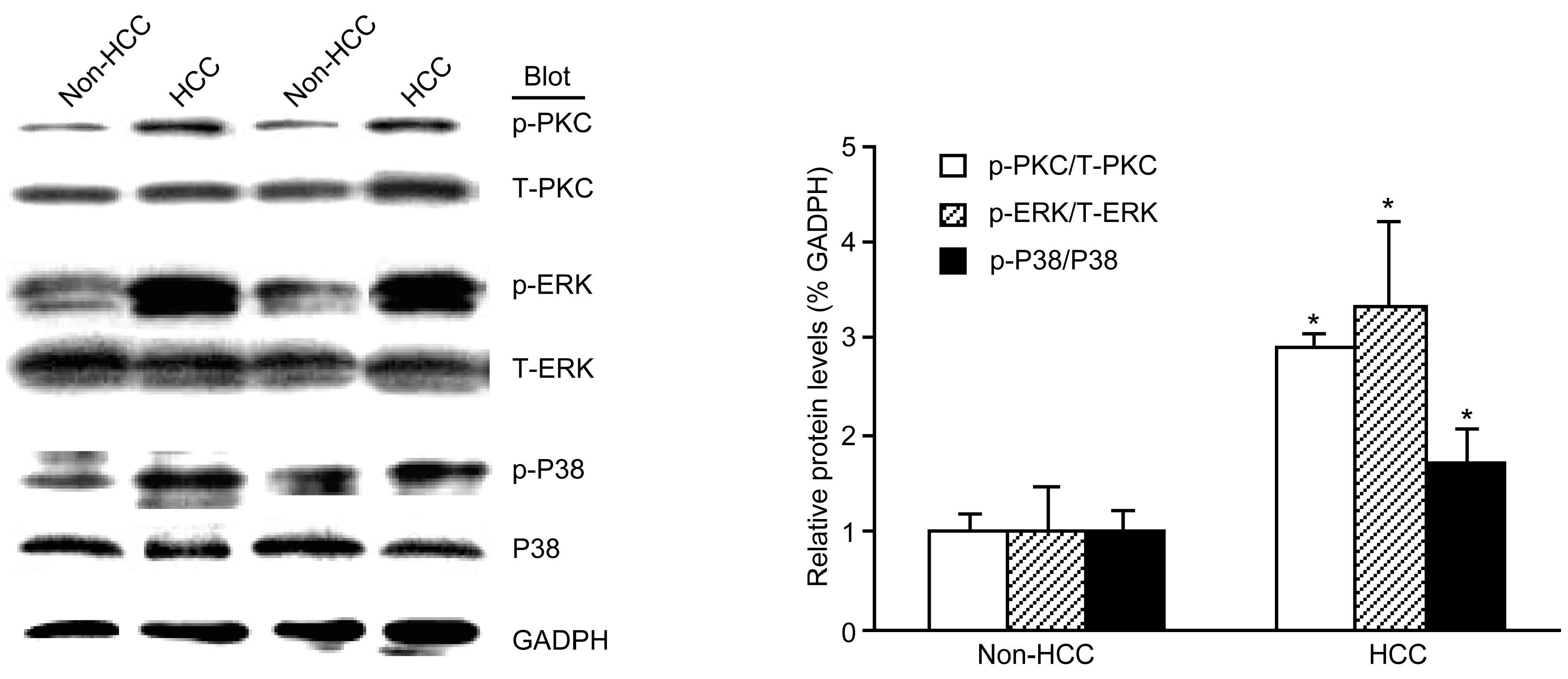
2.3. The Phosphorylation of PKC, ERK, and p38 MAPK Was Increased in HCC
2.4. Up-Regulation of the UII/UT System in BEL-7402 Cells


2.5. UII Induced the Phosphorylation of PKC, ERK, and p38 MAPK in BEL-7402 Cells

2.6. UII Increased the Proliferation of BEL-7402 Cells via Phosphorylation of PKC, ERK, and p38 MAPK

2.7. Discussion
3. Experimental Section
3.1. Patients
3.2. Materials
3.3. Cell Culture and Treatment
3.4. Quantitative Real-Time PCR Analysis
3.5. Western Blot Analysis
3.6. Immunohistochemistry Assay
3.7. BrdU Incorporation
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Coulouarn, Y.; Lihrmann, I.; Jegou, S.; Anouar, Y.; Tostivint, H.; Beauvillain, J.C.; Conlon, J.M.; Bern, H.A.; Vaudry, H. Cloning of the cDNA encoding the urotensin II precursor in frog and human reveals intense expression of the urotensin II gene in motoneurons of the spinal cord. Proc. Natl. Acad. Sci. USA 1998, 95, 15803–15808. [Google Scholar] [CrossRef] [PubMed]
- Pearson, D.; Shively, J.E.; Clark, B.R.; Geschwind, I.I.; Barkley, M.; Nishioka, R.S.; Bern, H.A. Urotensin II: A somatostatin-like peptide in the caudal neurosecretory system of fishes. Proc. Natl. Acad. Sci. USA 1980, 77, 5021–5024. [Google Scholar] [CrossRef] [PubMed]
- Coulouarn, Y.; Jegou, S.; Tostivint, H.; Vaudry, H.; Lihrmann, I. Cloning, sequence analysis and tissue distribution of the mouse and rat urotensin II precursors. FEBS Lett. 1999, 457, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Ames, R.S.; Sarau, H.M.; Chambers, J.K.; Willette, R.N.; Aiyar, N.V.; Romanic, A.M.; Louden, C.S.; Foley, J.J.; Sauermelch, C.F.; Coatney, R.W.; et al. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature 1999, 401, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Onan, D.; Hannan, R.D.; Thomas, W.G. Urotensin II: The old kid in town. Trends Endocrinol. Metab. 2004, 15, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Totsune, K.; Kitamuro, T.; Sone, M.; Murakami, O.; Shibahara, S. Three vasoactive peptides, endothelin-1, adrenomedullin and urotensin-II, in human tumour cell lines of different origin: Expression and effects on proliferation. Clin. Sci. 2002, 103, 35S–38S. [Google Scholar] [PubMed]
- Kusuhara, M.; Yamaguchi, K.; Nagasaki, K.; Hayashi, C.; Suzaki, A.; Hori, S.; Handa, S.; Nakamura, Y.; Abe, K. Production of endothelin in human cancer cell lines. Cancer Res. 1990, 50, 3257–3261. [Google Scholar] [PubMed]
- Takahashi, K.; Yoshinoya, A.; Murakami, O.; Totsune, K.; Shibahara, S. Production and secretion of two vasoactive peptides, adrenomedullin and endothelin-1, by cultured human adrenocortical carcinoma cells. Peptides 2000, 21, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Totsune, K.; Murakami, O.; Arihara, Z.; Noshiro, T.; Hayashi, Y.; Shibahara, S. Expression of urotensin II and its receptor in adrenal tumors and stimulation of proliferation of cultured tumor cells by urotensin II. Peptides 2003, 24, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.P.; Liu, G.Q.; Li, H.Z.; Fan, X.R.; Liu, D.M.; Tong, A.L.; Zheng, X; Liu, C. The effects of urotensin-II on proliferation of pheochromocytoma cells and mRNA expression of urotensin-II and its receptor in pheochromocytoma tissues. Ann. N.Y. Acad. Sci. 2006, 1073, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Zappavigna, S.; Romano, M.; Grieco, P.; Luce, A.; Marra, M.; Gravina, A.G.; Stiuso, P.; D’Armiento, F.P.; Vitale, G.; et al. Urotensin-II receptor is over-expressed in colon cancer cell lines and in colon carcinoma in humans. Eur. J. Clin. Investig. 2014, 44, 285–294. [Google Scholar] [CrossRef]
- Wang, H.; Dong, K.; Xue, X.; Feng, P.; Wang, X. Elevated expression of urotensin II and its receptor in diethylnitrosamine-mediated precancerous lesions in rat liver. Peptides 2011, 32, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T.; Thun, M.J. Cancer statistics, 2008. CA Cancer J. Clin. 2008, 58, 71–96. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, A.; Catt, K.J. Endothelins as autocrine regulators of tumor cell growth. Trends Endocrinol. Metab. 1998, 9, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, P.; Bousette, N.; Giaid, A. Urotensin-II and cardiovascular remodeling. Peptides 2008, 29, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, C.; Nunez, M.T. Calcium, iron and neuronal function. IUBMB Life 2007, 59, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Rozengurt, E. Mitogenic signaling pathways induced by G protein-coupled receptors. J. Cell. Physiol. 2007, 213, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Iglewski, M.; Grant, S.R. Urotensin II-induced signaling involved in proliferation of vascular smooth muscle cells. Vasc. Health Risk Manag. 2010, 6, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.T.; Hsieh, Y.H.; Hsieh, Y.S.; Liu, J.Y. Reduction of PKC alpha decreases cell proliferation, migration, and invasion of human malignant hepatocellular carcinoma. J. Cell. Biochem. 2008, 103, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, D.; Albertin, G.; Ribatti, D. Urotensin-II as an angiogenic factor. Peptides 2010, 31, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, D.; Albertin, G.; Oselladore, B.; Sorato, E.; Rebuffat, P.; Mascarin, A.; Ribatti, D. The pro-angiogenic activity of urotensin-II on human vascular endothelial cells involves ERK1/2 and PI3K signaling pathways. Regul. Pept. 2010, 162, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, T.P.; Tian, C.; Jia, L.X.; Du, J.; Li, H.H. Carboxyl terminus of heat shock protein 70-interacting protein inhibits angiotensin II-induced cardiac remodeling. Am. J. Hypertens. 2012, 25, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Yang, H.; Han, Q.Y.; Li, N.; Jiang, X.; Tian, C.; Du, J.; Li, H.H. NADPH oxidases mediate a cellular “memory” of angiotensin II stress in hypertensive cardiac hypertrophy. Free Radic. Biol. Med. 2013, 65, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.-T.; Wang, P.-Y.; Shi, Z.-M.; Dong, K.; Feng, P.; Wang, H.-X.; Wang, X.-J. Up-Regulation of Urotensin II and Its Receptor Contributes to Human Hepatocellular Carcinoma Growth via Activation of the PKC, ERK1/2, and p38 MAPK Signaling Pathways. Molecules 2014, 19, 20768-20779. https://doi.org/10.3390/molecules191220768
Yu X-T, Wang P-Y, Shi Z-M, Dong K, Feng P, Wang H-X, Wang X-J. Up-Regulation of Urotensin II and Its Receptor Contributes to Human Hepatocellular Carcinoma Growth via Activation of the PKC, ERK1/2, and p38 MAPK Signaling Pathways. Molecules. 2014; 19(12):20768-20779. https://doi.org/10.3390/molecules191220768
Chicago/Turabian StyleYu, Xiao-Tong, Peng-Yan Wang, Zheng-Ming Shi, Kun Dong, Ping Feng, Hong-Xia Wang, and Xue-Jiang Wang. 2014. "Up-Regulation of Urotensin II and Its Receptor Contributes to Human Hepatocellular Carcinoma Growth via Activation of the PKC, ERK1/2, and p38 MAPK Signaling Pathways" Molecules 19, no. 12: 20768-20779. https://doi.org/10.3390/molecules191220768



