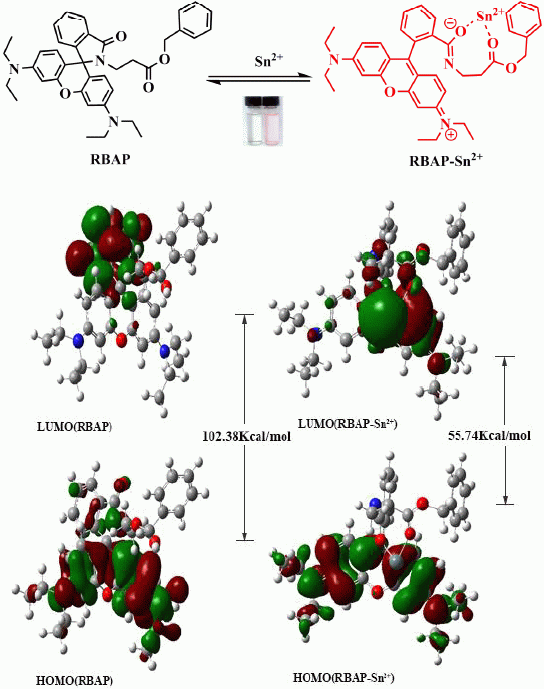
RBAP, a Rhodamine B-Based Derivative: Synthesis, Crystal Structure Analysis, Molecular Simulation, and Its Application as a Selective Fluorescent Chemical Sensor for Sn2+
Abstract
:1. Introduction

2. Results and Discussion
2.1. Synthesis of RBAP

2.2. X-ray Crystallographic Analysis


2.3. pH Response of RBAP


2.4. UV-Vis Titration of RBAP with Metal Ions



2.5. Fluorescence Titration of RBAP with Metal Ions
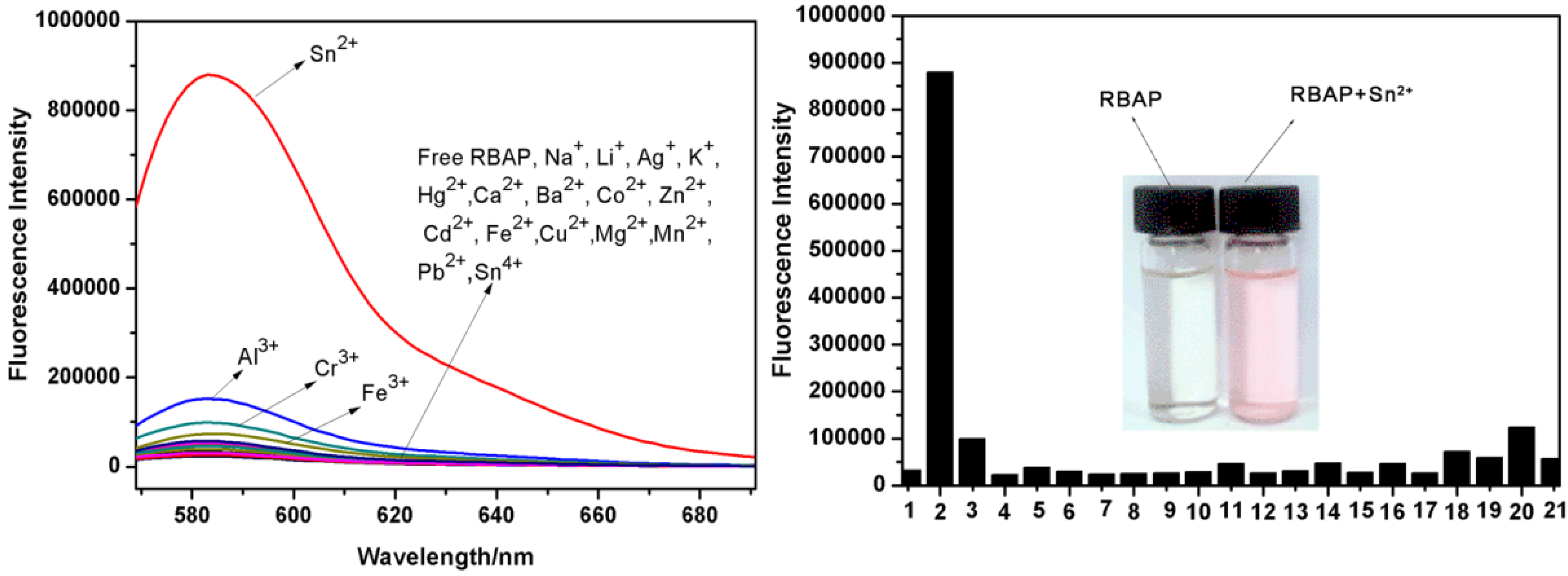

2.6. Fluorescence Titration of RBAP with Sn2+

2.7. NMR Study of RBAP with Sn2+

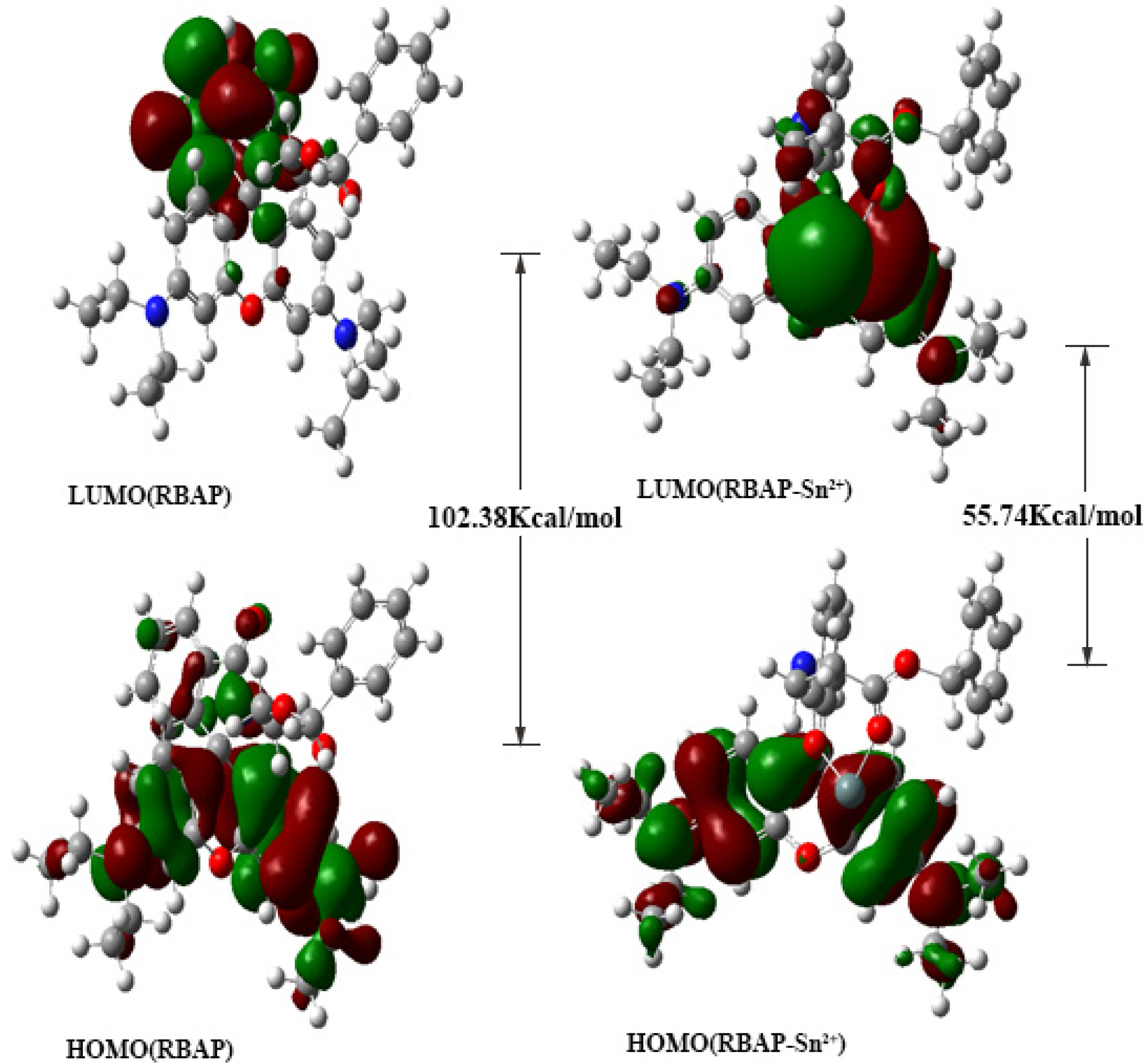
2.8. Density Functional Theory (DFT) Calculations

3. Experimental Section
3.1. Materials and General Methods
3.2. Synthesis of 3-(Benzyloxy)-3-oxopropan-1-aminium chloride (2)
3.3. Synthesis of Benzyl 3-(3',6'-bis(diethylamino)-3-oxospiro[isoindoline-1,9'-xanthen]-2-yl) propan oate (RBAP)
3.4. Experimental Procedure for X-ray Crystallographic Analysis
3.5. Stock Solution Preparation for Spectral Detection
3.6. Computational Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guo, X.F.; Qian, X.H.; Jia, L.H. A highly selective and sensitive fluorescent chemosensor for Hg2+ in neutral buffer aqueous solution. J. Am. Chem. Soc. 2004, 126, 2272–2273. [Google Scholar]
- Shiraishi, Y.; Sumiya, S.; Kohno, Y.; Hirai, T. A Rhodamine-cyclen conjugate as a highly sensitive and selective fluorescent chemosensor for Hg(II). J. Org. Chem. 2008, 73, 8571–8574. [Google Scholar] [CrossRef]
- Tian, M.Z.; Hu, M.M.; Fan, J.L.; Peng, X.J.; Wang, J.-Y.; Sun, S.G.; Zhang, R. Rhodamine-based “turn-on” fluorescent probe for Cu(II) and its fluorescence imaging in living cells. Bioorg. Med. Chem. Lett. 2013, 23, 2916–2919. [Google Scholar]
- Wu, D.; Huang, W.; Duan, C.; Lin, Z.; Meng, Q. Highly sensitive fluorescent probe for selective detection of Hg2+ in DMF aqueous media. Inorg. Chem. 2007, 46, 1538–1540. [Google Scholar]
- Yin, S.; Leen, V.; van Snick, S.; Boens, N.; Dehaen, W. A highly sensitive, selective, colorimetric and near-infrared fluorescent turn-on chemosensor for Cu2+ based on BODIPY. Chem. Commun. 2010, 46, 6329–6331. [Google Scholar]
- Goswami, S.; Aich, K.; Das, S.; Das, A.K.; Manna, A.; Halder, S. A highly selective and sensitive probe for colorimetric and fluorogenic detection of Cd2+ in aqueous media. Analyst 2013, 138, 1903–1907. [Google Scholar]
- Goswami, S.; Das, S.; Aich, K.; Sarkar, D.; Mondal, T.K.; Quah, C.K.; Fun, H.K. CHEF induced highly selective and sensitive turn-on fluorogenic and colorimetric sensor for Fe3+. Dalton Trans. 2013, 42, 15113–15119. [Google Scholar]
- Bannon, D.I.; Chisolm, J.J. Anodic stripping voltammetry compared with graphite furnace atomic absorption spectrophotometry for blood lead analysis. Clin. Chem. 2001, 47, 1703–1704. [Google Scholar]
- Hill, S.J.; Chenery, S.; Dawson, J.B.; Evans, E.H.; Fisher, A.; Price, W.J.; Smith, C.M.M.; Sutton, K.L.; Tyson, J.F. Advances in atomic emission, absorption and fluorescence spectrometry, and related techniques. J. Anal. At. Spectrom. 2000, 15, 763–805. [Google Scholar] [CrossRef]
- Resano, M.; Vanhaecke, F.; de Loos-Vollebregt, M.T.C. Electrothermal vaporization for sample introduction in atomic absorption, atomic emission and plasma mass spectrometry-a critical review with focus on solid sampling and slurry analysis. J. Anal. At. Spectrom. 2008, 23, 1450–1475. [Google Scholar] [CrossRef]
- Vadillo, J.M.; Garcia, C.C.; Alcantara, J.F.; Laserna, J.J. Thermal-to-plasma transitions and energy threshold in laser ablated metals monitored by atomic emission/mass spectrometry coincidence analysis. Spectrochim. Acta Part B At. Spectrosc. 2005, 60, 948–954. [Google Scholar] [CrossRef]
- Romao, W.; Lalli, P.M.; Franco, M.F.; Sanvido, G.; Schwab, N.V.; Lanaro, R.; Costa, J.L.; Sabino, B.D.; Bueno, M.I.M.S.; de Sa, G.F.; et al. Chemical profile of meta-chlorophenylpiperazine (m-CPP) in ecstasy tablets by easy ambient sonic-spray ionization, X-ray fluorescence, ion mobility mass spectrometry and NMR. Anal. Bioanal. Chem. 2011, 400, 3053–3064. [Google Scholar] [CrossRef]
- Van Meel, K.; Smekens, A.; Behets, M.; Kazandjian, P.; van Grieken, R. Determination of platinum, palladium, and rhodium in automotive catalysts using high-energy secondary target X-ray fluorescence spectrometry. Anal. Chem. 2007, 79, 6383–6389. [Google Scholar] [CrossRef]
- Forzani, E.S.; Zhang, H.Q.; Chen, W.; Tao, N.J. Detection of heavy metal ions in drinking water using a high-resolution differential surface plasmon resonance sensor. Environ. Sci. Technol. 2005, 39, 1257–1262. [Google Scholar] [CrossRef]
- Rojas, E.; Herrera, L.A.; Poirier, L.A.; Ostrosky-Wegman, P. Are metals dietarycarcinogens? Mutat. Res. 1999, 443, 157–181. [Google Scholar] [CrossRef]
- Unal, U.; Somer, G. Simultaneous determination of trace Sn(II) and Sn(IV) using differential pulse polarography and application. Turkish J. Chem. 2011, 35, 73–85. [Google Scholar]
- Braga, A.C.S.; Oliveira, M.B.N.; Feliciano, G.D.; Reiniger, I.W.; Oliveira, J.F.; Silva, C.R.; Bernardo, M. The effect of drugs on the labeling of blood elements with technetium-99m. Curr. Pharm. Des. 2000, 6, 1179–1191. [Google Scholar] [CrossRef]
- Viau, C.M.; Guecheva, T.N.; Sousa, F.G.; Pungartnik, C.; Brendel, M.; Saffi, J.; Pegas Henriques, J.A. SnCl2-induced DNA damage and repair inhibition of MMS-caused lesions in V79 Chinese hamster fibroblasts. Arch. Toxicol. 2009, 83, 769–775. [Google Scholar] [CrossRef]
- Bao, X.; Liu, D.; Jin, Y.; Liu, X.; Jiang, W. Design and synthesis of a novel Rhodamine B [2] rotaxane. RSC Adv. 2013, 3, 6783–6786. [Google Scholar] [CrossRef]
- Biswal, B.; Bag, B. Preferences of rhodamine coupled (aminoalkyl)-piperazine probes towards Hg(II) ion and their FRET mediated signaling. Org. Biomol. Chem. 2013, 11, 4975–4992. [Google Scholar] [CrossRef]
- Duong Tuan, Q.; Kim, J.S. Chromogenic chemodosimeters for heavy metal ion detection in solution and biospecimens. Chem. Rev. 2010, 110, 6280–6301. [Google Scholar] [CrossRef]
- Kim, H.N.; Lee, M.H.; Kim, H.J.; Kim, J.S.; Yoon, J. A new trend in rhodamine-based chemosensors: Application of spirolactam ring-opening to sensing ions. Chem. Soc. Rev. 2008, 37, 1465–1472. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Jang, Y.J.; Lee, Y.J.; Kim, K.M.; Seo, M.S.; Nam, W.; Yoon, J. A highly selective fluorescent chemosensor for Pb2+. J. Am. Chem. Soc. 2005, 127, 10107–10111. [Google Scholar]
- Liu, J.; Wu, D.; Yan, X.; Guan, Y. Naked-eye sensor for rapid determination of mercury ion. Talanta 2013, 116, 563–568. [Google Scholar] [CrossRef]
- Ni, J.; Li, Q.; Li, B.; Zhang, L. A novel fluorescent probe based on rhodamine B derivative for highly selective and sensitive detection of mercury(II) ion in aqueous solution. Sens. Actuat. B Chem. 2013, 186, 278–285. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, Z.; Huang, K.; Yu, M.; Li, F.; Yi, T.; Huang, C. Multisignaling optical-electrochemical sensor for Hg2+ based on a rhodamine derivative with a ferrocene unit. Org. Lett. 2007, 9, 4729–4732. [Google Scholar]
- Yu, M.; Shi, M.; Chen, Z.; Li, F.; Li, X.; Gao, Y.; Xu, J.; Yang, H.; Zhou, Z.; Yi, T.; et al. Highly sensitive and fast responsive fluorescence turn-on chemodosimeter for Cu(2+) and its application in live cell imaging. Chemistry 2008, 14, 6892–6900. [Google Scholar] [CrossRef]
- Chen, X.; Pradhan, T.; Wang, F.; Kim, J.S.; Yoon, J. Fluorescent chemosensors based on spiroring-opening of xanthenes and related derivatives. Chem. Rev. 2012, 112, 1910–1956. [Google Scholar] [CrossRef]
- Goswami, S.; Manna, A.; Maity, A.K.; Paul, S.; Das, A.K.; Das, M.K.; Saha, P.; Quah, C.K.; Fun, H.-K. Selective detection and bio-imaging of Pd2+ with novel “C-CN” bond cleavage of cyano-rhodamine, cyanation with diaminomaleonitrile. Dalton Trans. 2013, 42, 12844–12848. [Google Scholar] [CrossRef]
- Mahapatra, A.K.; Manna, S.K.; Mandal, D.; Das Mukhopadhyay, C. Highly sensitive and selective rhodamine-based “off on” reversible chemosensor for tin (sn4+) and imaging in living cells. Inorg. Chem. 2013, 52, 10825–10834. [Google Scholar]
- Liu, D.; Pang, T.; Ma, K.; Jiang, W.; Bao, X. A new highly sensitive and selective fluorescence chemosensor for Cr3+ based on rhodamine B and a 4,13-diaza-18-crown 6-ether conjugate. RSC Adv. 2014, 4, 2563–2567. [Google Scholar]
- Sample Availability: Samples of the compounds 2 and RBAP are available from the authors.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bao, X.; Cao, X.; Nie, X.; Jin, Y.; Zhou, B. RBAP, a Rhodamine B-Based Derivative: Synthesis, Crystal Structure Analysis, Molecular Simulation, and Its Application as a Selective Fluorescent Chemical Sensor for Sn2+. Molecules 2014, 19, 7817-7831. https://doi.org/10.3390/molecules19067817
Bao X, Cao X, Nie X, Jin Y, Zhou B. RBAP, a Rhodamine B-Based Derivative: Synthesis, Crystal Structure Analysis, Molecular Simulation, and Its Application as a Selective Fluorescent Chemical Sensor for Sn2+. Molecules. 2014; 19(6):7817-7831. https://doi.org/10.3390/molecules19067817
Chicago/Turabian StyleBao, Xiaofeng, Xiaowei Cao, Xuemei Nie, Yanyan Jin, and Baojing Zhou. 2014. "RBAP, a Rhodamine B-Based Derivative: Synthesis, Crystal Structure Analysis, Molecular Simulation, and Its Application as a Selective Fluorescent Chemical Sensor for Sn2+" Molecules 19, no. 6: 7817-7831. https://doi.org/10.3390/molecules19067817