Synthesis, Spectroscopic and Theoretical Studies of New Dimeric Quaternary Alkylammonium Conjugates of Sterols
Abstract
:1. Introduction

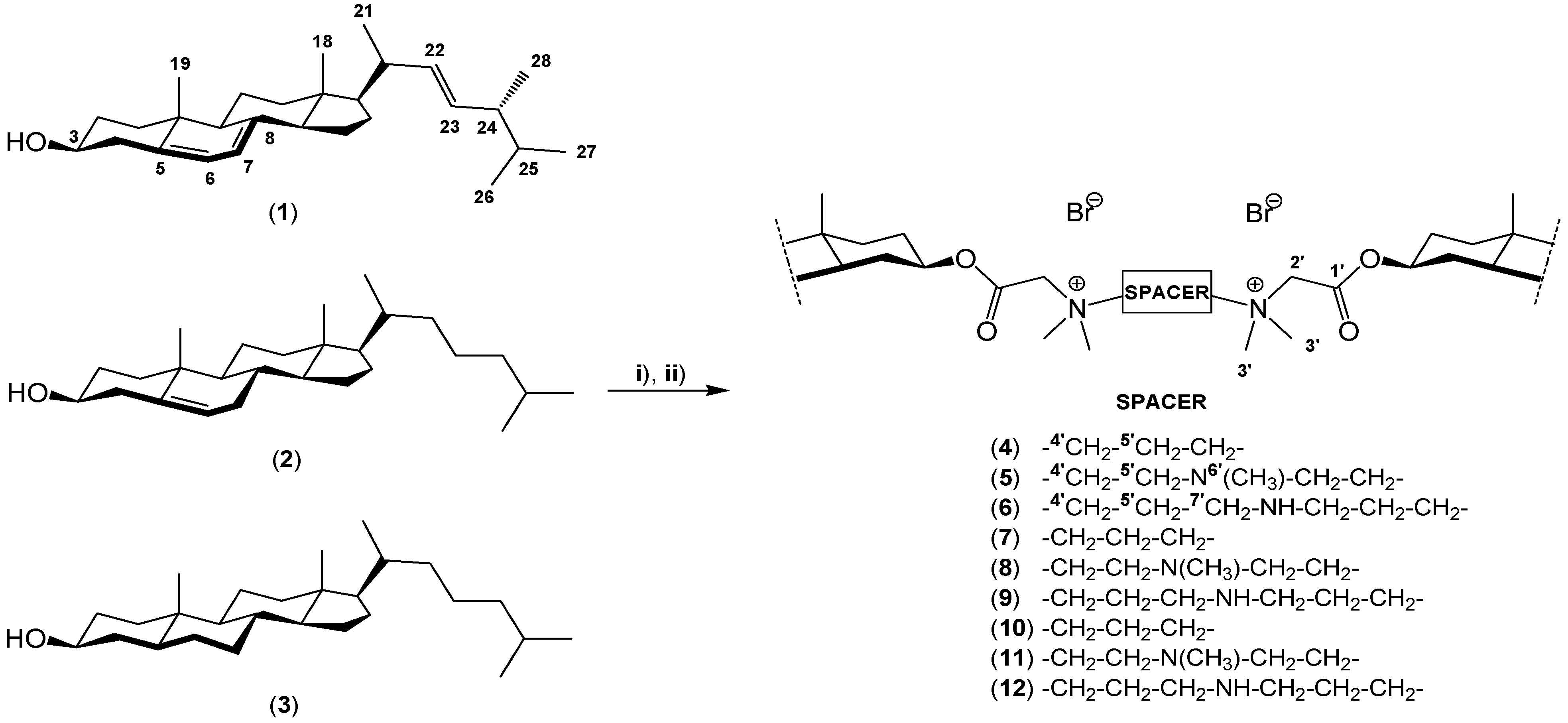
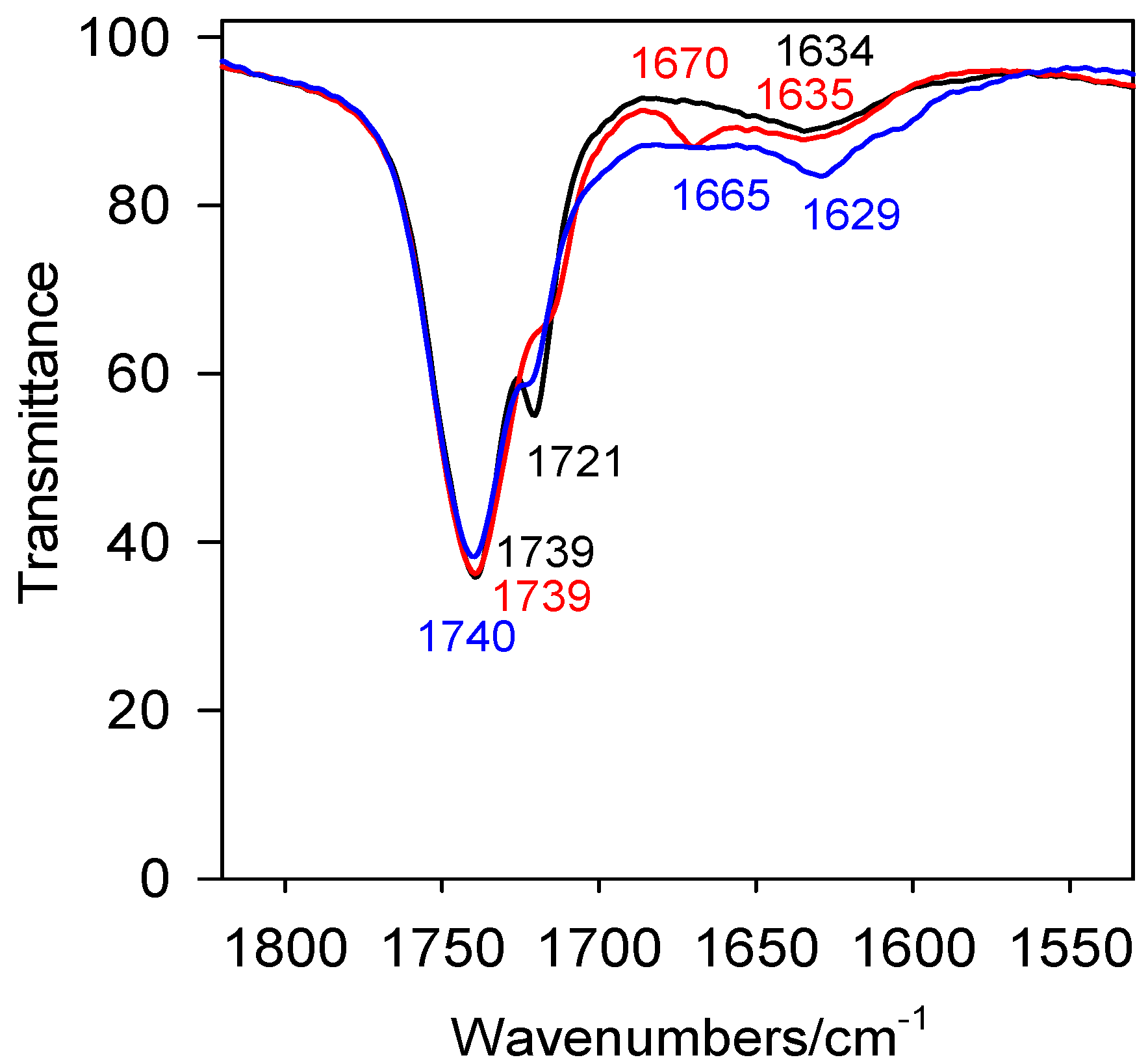
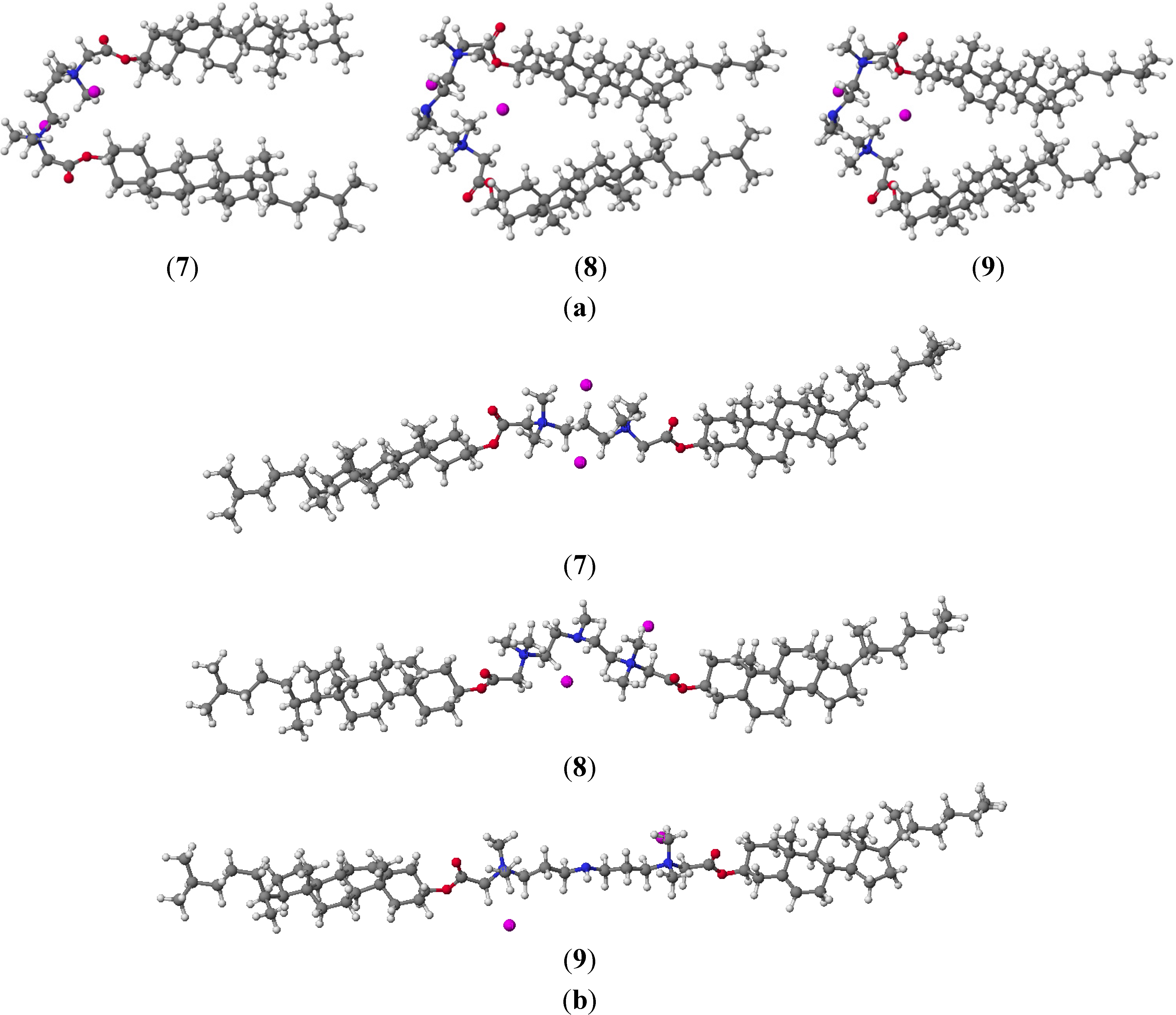
2. Results and Discussion

| Carbon | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|
| 1' | 163.70 | 163.89 | 164.07 | 163.57 | 163.75 | 163.95 | 163.59 | 163.87 | 163.99 |
| 2' | 62.71 | 61.91 | 65.61 | 62.07 | 61.54 | 65.59 | 62.24 | 61.49 | 65.58 |
| 3' | 51.58 | 52.15 | 50.82 | 51.72 | 52.18 | 50.81 | 51.49 | 52.21 | 50.82 |
| 4' | 62.07 | 61.88 | 61.62 | 61.66 | 61.52 | 61.58 | 62.24 | 61.48 | 61.59 |
| 5' | – | 56.92 | – | – | 55.96 | – | – | 54.02 | – |
| 6' | – | 49.67 | – | – | 49.82 | – | – | 49.88 | – |
| 7' | – | – | 57.01 | – | – | 56.60 | – | – | 54.02 |
| 3 | 76.77 | 76.56 | 76.55 | 77.19 | 76.67 | 77.13 | 77.15 | 76.97 | 77.20 |
| 5 | 139.75 | 139.73 | 139.87 | 138.56 | 138.55 | 138.74 | – | – | – |
| 6 | 123.26 | 123.21 | 123.30 | 123.37 | 138.55 | 123.38 | – | – | – |
| 7 | 118.08 | 118.02 | 118.11 | – | – | – | – | – | – |
| 8 | 150.47 | 150.45 | 150.55 | – | – | – | – | – | – |
| 18 | 15.68 | 15.63 | 15.79 | 11.65 | 11.66 | 11.77 | 11.86, 11.96 | 11.88, 11.99 | 11.92, 12.12 |
| 19 | 18.08 | 18.03 | 18.23 | 18.99 | 19.01 | 19.15 | 18.45 | 18.48 | 18.58 |
| 21 | 19.83 | 19.77 | 19.92 | 18.50 | 18.51 | 18.64 | 21.04 | 21.07 | 21.13 |
| 22 | 135.33 | 135.30 | 135.42 | – | – | – | – | – | – |
| 23 | 132.02 | 131.98 | 132.07 | – | – | – | – | – | – |
| 26/27 | 19.51 | 19.45 | 19.60 | 22.56 | 22.59 | 22.47 | 22.57 | 22.58 | 22.47 |
| 28 | 17.52 | 17.47 | 17.62 | – | – | – | – | – | – |
| Compound | HOF [kcal/mol] | HOF syn Conformer [kcal/mol] | HOF anti Conformer [kcal/mol] | ΔHOF syn [kcal/mol] | ΔHOF anti [kcal/mol] |
|---|---|---|---|---|---|
| 1 | −97.1208 | - | - | - | - |
| 2 | −140.1058 | - | - | - | - |
| 3 | −162.7945 | - | - | - | - |
| 4 | - | −262.2720 | −255.9961 | −165.1512 | −158.8753 |
| 5 | - | −250.2337 | −250.1800 | −153.1129 | −153.0592 |
| 6 | - | −276.4203 | −257.6516 | −179.2995 | −160.5308 |
| 7 | - | −345.4121 | −341.1838 | −205.3063 | −201.0780 |
| 8 | - | −338.9490 | −332.2815 | −198.8432 | −192.1757 |
| 9 | - | −358.2366 | −342.9308 | −218.1308 | −202.8250 |
| 10 | - | −393.0944 | −386.6933 | −230.2990 | −223.8988 |
| 11 | - | −380.3771 | −377.8571 | −217.5826 | −215.0626 |
| 12 | - | −407.2888 | −388.2125 | −244.4943 | −225.4180 |


| Focal Predicted Activity (PA > 80) | Compounds | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Glyceryl-ether monooxygenase inhibitor | 89 | 89 | 88 | 92 | 92 | 91 | 95 | 95 | 94 |
| Cholesterol antagonist | 81 | 85 | – | 87 | 89 | 82 | 82 | 86 | – |
| Antihypercholesterolemic | 88 | 83 | 86 | 85 | 80 | 83 | - | 94 | – |
| Acylcarnitine hydrolase inhibitor | – | - | - | 85 | 80 | - | 96 | - | 93 |
| Oxidoreductase inhibitor | 87 | 86 | 85 | - | - | - | - | - | - |
| Alkylacetylglycerophosphatase inhibitor | - | - | - | - | - | - | 90 | 87 | 83 |
| Alkenylglycerophosphocholine hydrolase inhibitor | - | - | - | - | - | - | 88 | 82 | 80 |
| Alcohol O-acetyltransferase inhibitor | 91 | 90 | 90 | - | - | - | - | - | - |
3. Experimental Section
3.1. General Information
3.2. Synthesis: General Procedure for the Synthesis of Quaternary Ammonium C Onjugates of Sterols
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nicolaou, K.C.; Montagnon, T. Molecules that Changed the World; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 79–90. [Google Scholar]
- Dewick, P.M. Medicinal Natural Products A Biosynthetic Approach, 3rd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 275–277. [Google Scholar]
- Koskinen, A.M.P. Asymmetric Synthesis of Natural Products; John Wiley & Sons, Ltd.: Chichester, UK, 2012; pp. 235–244. [Google Scholar]
- Fieser, L.F.; Fieser, M. Steroids; Reinhold Publishing Corporation: New York, NY, USA, 1959; pp. 53–90, 341–364. [Google Scholar]
- Templeton, W. An Introduction to the Chemistry of Terpenoids and Steroids; Butterworths: London, UK, 1969; pp. 158–190. [Google Scholar]
- Lednicer, D. Steroid Chemistry at a Glance; John Wiley & Sons, Ltd.: Chichester, UK, 2011. [Google Scholar]
- Parish, E.J.; Nes, W.D. Biochemistry and Function of Sterols; CRC-Press : Boca Raton, FL, USA, 1997. [Google Scholar]
- Schaller, H. The role of sterols in plant growth and development. Prog. Lipid Res. 2003, 42, 163–175. [Google Scholar] [CrossRef]
- Jäpelt, R.B.; Jakobsem, J. Vitamin D in plants: A review of occurrence, analysis, and biosynthesis. Front. Plant. Sci. 2013, 4, 1–20. [Google Scholar]
- Hanson, J.R. The Chemistry of Fungi; Royal Society of Chemistry: Cambridge, UK, 2008; pp. 1–18. [Google Scholar]
- Vance, D.E.; van den Bosch, H. Cholesterol in the year 2000. Biochim. Biophys. Acta 2000, 1529, 1–8. [Google Scholar] [CrossRef]
- Ikan, R. Natural Products a Laboratory Guide, 2nd ed.; Academic Press: London, UK, 1991; pp. 154–159. [Google Scholar]
- Bloch, K. Sterol molecule: Structure, biosynthesis, and function. Steroids 1997, 57, 378–383. [Google Scholar] [CrossRef]
- Bloch, K. 50 Years ago, the structure of cholesterol and of the bile acids. Trends Biochem. Sci. 1982, 7, 334–336. [Google Scholar]
- Murry, K.R.; Granner, D.K.; Mayes, P.A.; Rodwell, V.W. Harper’s Biochemistry, 26th ed.; McGraw-Hill: New York, NY, USA, 1996; pp. 219–230. [Google Scholar]
- Risley, J.M. Cholesterol biosynthesis: Lanosterol to cholesterol. J. Chem. Educ. 2002, 79, 377–384. [Google Scholar] [CrossRef]
- Hanukoglu, I. Steroidogenic enzymes: Structure, function, and role in regulation of steroid hormone biosynthesis. J. Steroid Biochem. Mol. Biol. 1992, 43, 779–804. [Google Scholar] [CrossRef]
- Nagrady, T.; Weaver, D.F. Medicinal Chemistry A Molecular and Biochemical Approach, 3rd ed.; Oxford University Press: New York, NY, USA, 2005; pp. 316–320. [Google Scholar]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 458–470, 502–529. [Google Scholar]
- Zana, R.; Xia, J. Gemini Surfactants Synthesis,Interfacial and Solution-Phase Behavior,and Applications; Marcel Dekker: New York, NY, USA, 2004; pp. 1–5. [Google Scholar]
- Menger, F.M.; Keiper, J.S. Gemini Surfactants. Angew. Chem. Int. Ed. 2000, 39, 1906–1920. [Google Scholar] [CrossRef]
- Menger, F.M.; Littau, C.A. Gemini Surfactants: Synthesis and Properties. J. Am. Chem. Soc. 1991, 113, 1451–1452. [Google Scholar] [CrossRef]
- Hait, S.K.; Moulik, S.P. Gemini surfactants: A distinct class of self-assembling molecules. Curr. Sci. 2002, 82, 1101–1111. [Google Scholar]
- Holmberg, K.; Jönsson, B.; Kronberg, B.; Lindman, B. Surfactants and Polymers in Aqueous Solution, 2nd ed.; John Wiley & Sons: Chichester, UK, 2003; pp. 227–261. [Google Scholar]
- Brycki, B. Gemini Alkylammonium Salts as Biodeterioration Inhibitors. Pol. J. Microbiol. 2010, 59, 227–231. [Google Scholar]
- Pisárčik, M.; Devínsky, F.; Lacko, I. Critical micelle concentration, ionization degree and micellisation energy of cationic dimeric (gemini) surfactants in aqueous solution and in mixed micelles with anionic surfactant. Acta Facult. Pharm. Univ. Comen. 2003, 50, 119–131. [Google Scholar]
- Kuperkar, K.; Modi, J.; Patel, K. Surface-Active Properties and Antimicrobial Study of Conventional Cationic and Synthesized Symmetrical Gemini Surfactants. J. Surfact. Deterg. 2012, 15, 107–115. [Google Scholar] [CrossRef]
- Shukla, D.; Tyagi, V.K. Cationic Gemini Surfactants: A Review. J. Oleo. Sci. 2006, 55, 381–390. [Google Scholar] [CrossRef]
- Para, G.; Hamerska-Dudra, A.; Wilk, K.A.; Warszyński, P. Surface activity of cationic surfactants, influence of molecular structure. Colloid Surf. A 2010, 365, 215–221. [Google Scholar] [CrossRef]
- Brycki, B.; Kowalczyk, I.; Koziróg, A. Synthesis, Molecular Structure, Spectral Properties and Antifungal Activity of Polymethylene-α,ω-bis(N, N-dimethyl-N-dodecyloammonium bromides. Molecules 2011, 16, 319–335. [Google Scholar] [CrossRef]
- Ng, C.K.L.; Obando, D.; Widmer, F.; Wright, L.C.; Sorrell, T.C.; Jolliffe, K.A. Correlation of Antifungal Activity with Fungal Phospholipase Inhibition Using a Series of Bisquaternary Ammonium Salts. J. Med. Chem. 2006, 49, 811–816. [Google Scholar] [CrossRef]
- Laatiris, A.; El Achouri, M.; Infante, M.R.; Bensouda, Y. Antibacterial activity, structure and CMC relationships of alkanediyl α,ω-bis(dimethylammonium bromide) surfactants. Microbiol. Res. 2008, 163, 645–650. [Google Scholar] [CrossRef]
- Diz, M.; Manresa, A.; Pinazo, A.; Erra, P.; Infante, M.R. Synthesis, Surface Active Properties and Antimicrobial Activity of New Bis Quaternary Ammonium Compounds. J. Chem. Soc. Perkin. Trans. 1994, 2, 1871–1876. [Google Scholar]
- Fraise, A.P.; Maillard, J.-Y.; Sattar, S.A. Russell, Hugo & Ayliffe’s Principles and Practice of Disinfection, Preservation & Sterilization, 5th ed.; Wiley-Blackwell: Chichester, UK, 2013. [Google Scholar]
- Walker, E.B. Quaternary Ammonium Compounds. In Handbook of Topical Antimicrobials Industrial Applications in Consumer Products and Pharmaceuticals; Paulson, D.S., Ed.; Marcel Dekker: New York, NY, USA, 2002; pp. 99–117. [Google Scholar]
- Poroikov, V.V.; Druzhilovsky, D.; Vasilenko, V. Pharma Expert Predictive Services © 2011–2013,Version 2.0. Available online: http://www.pharmaexpert.ru/PASSOnline/ (accessed on 1 November 2013).
- Poroikov, V.V.; Filimonov, D.A.; Borodina, Y.V.; Lagunin, A.A.; Kos, A. Robustness of biological activity spectra predicting by computer program PASS for noncongeneric sets of chemical compounds. J. Chem. Inf. Comput. Sci. 2000, 40, 1349–1355. [Google Scholar] [CrossRef]
- Poroikov, V.V.; Filimonov, D.A. How to acquire new biological activities in old compounds by computer prediction. J. Comput. Aided Mol. Des. 2002, 16, 819–824. [Google Scholar] [CrossRef]
- Poroikov, V.V.; Filimonov, D.A. PASS: Prediction of Biological Activity Spectra for Substances. In Predictive Toxicology; Christopher, H., Ed.; Taylor and Francis: Boca Raton, FL, USA, 2005; pp. 459–478. [Google Scholar]
- Stepanchikova, A.V.; Lagunin, A.A.; Filimonov, D.A.; Poroikov, V.V. Prediction of biological activity spectra for substances: Evaluation on the diverse sets of drug-like structures. Curr. Med. Chem. 2003, 10, 225–233. [Google Scholar] [CrossRef]
- Brycki, B.; Koenig, H.; Kowalczyk, I.; Pospieszny, T. Synthesis, spectroscopic and semiempirical studies of new quaternary alkylammonium conjugates of sterols. Molecules 2013, 18, 14961–14976. [Google Scholar] [CrossRef]
- Brycki, B.; Koenig, H.; Kowalczyk, I.; Pospieszny, T. Synthesis, spectroscopic and theoretical studies of new quaternary N, N-dimethyl-3-phtalimidopropylammonium conjugates of sterols and bile acids. Molecules 2014, 19, 4212–4233. [Google Scholar]
- CAChe 5.04 User Guide, Fujitsu: Chiba, Japan, 2003.
- Stewart, J.J.P. Optimization of parameters for semiempirical methods. III Extension of PM3 to Be, Mg, Zn, Ga, Ge, As, Se, Cd, In, Sn, Sb, Te, Hg, Tl, Pb, and Bi. J. Comput. Chem. 1991, 12, 320–341. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods I. Method. J. Comput. Chem. 1989, 10, 209–220. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 4–12 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brycki, B.; Koenig, H.; Kowalczyk, I.; Pospieszny, T. Synthesis, Spectroscopic and Theoretical Studies of New Dimeric Quaternary Alkylammonium Conjugates of Sterols. Molecules 2014, 19, 9419-9434. https://doi.org/10.3390/molecules19079419
Brycki B, Koenig H, Kowalczyk I, Pospieszny T. Synthesis, Spectroscopic and Theoretical Studies of New Dimeric Quaternary Alkylammonium Conjugates of Sterols. Molecules. 2014; 19(7):9419-9434. https://doi.org/10.3390/molecules19079419
Chicago/Turabian StyleBrycki, Bogumił, Hanna Koenig, Iwona Kowalczyk, and Tomasz Pospieszny. 2014. "Synthesis, Spectroscopic and Theoretical Studies of New Dimeric Quaternary Alkylammonium Conjugates of Sterols" Molecules 19, no. 7: 9419-9434. https://doi.org/10.3390/molecules19079419




