Effects of Subacute Hypothyroidism on Metabolism and Growth-Related Molecules
Abstract
:1. Introduction
2. Results and Discussion
2.1. Changes in Body Weight and Metabolism


2.2. Effects of Hypothyroidism on Thyroid-Stimulating Hormone (TSH)
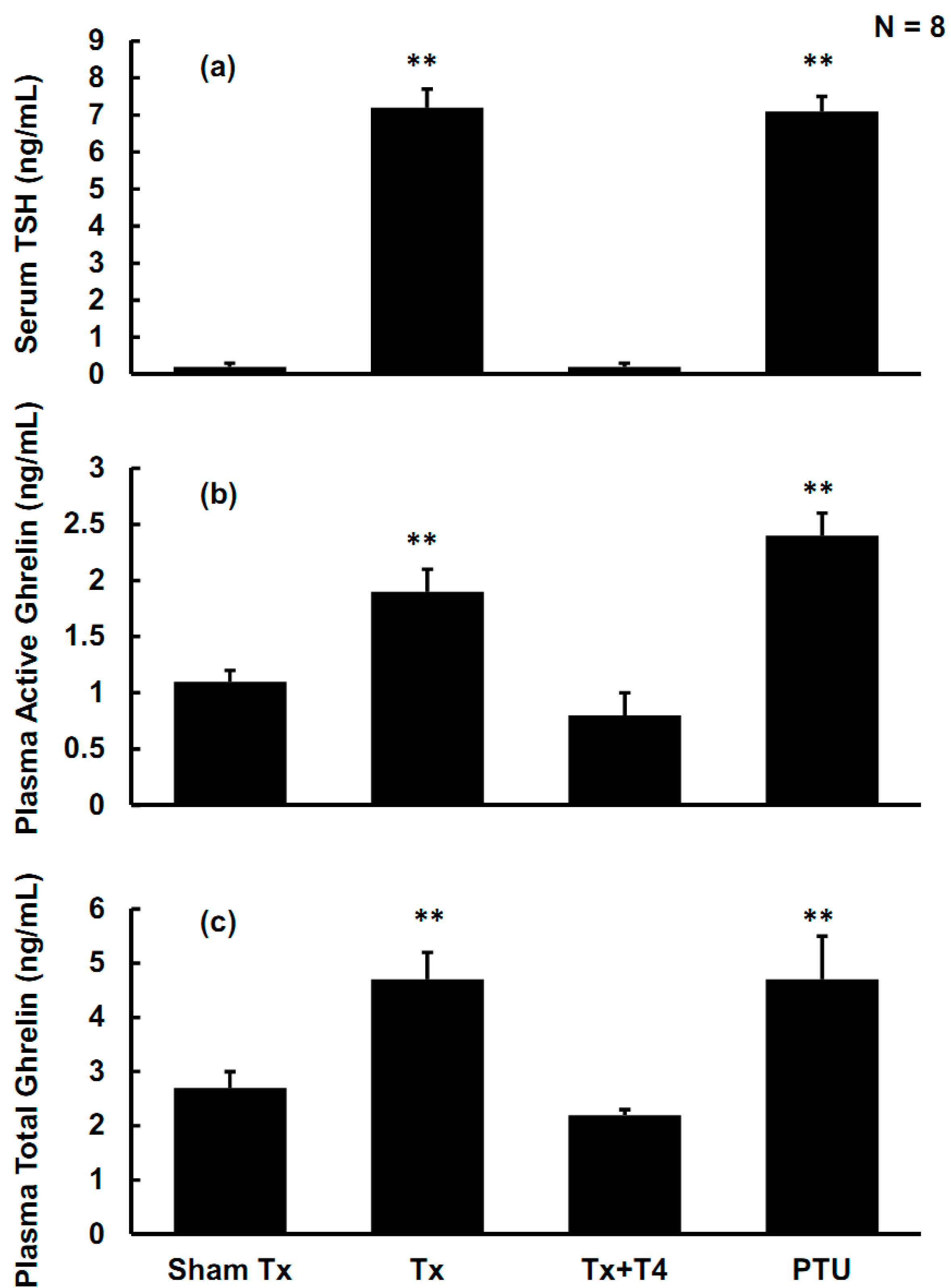
2.3. Effects of Hypothyroidism on Active Ghrelin, Total Ghrelin, and Ghrelin Receptors in the Anterior Pituitary (AP)

2.4. Effects of Hypothyroidism on Growth Hormone (GH) and Insulin-Like Growth Factor-1 (IGF-1)

2.5. Relationship among TSH, Active Ghrelin, Total Ghrelin, GH, and IGF-1

2.6. Relationship of Food Intake/Body Weight (BW) to Active Ghrelin, TSH, and IGF-1

2.7. Discussion
3. Experimental Section
3.1. Animals
3.2. Chemicals, Antibodies, and Assay Kits
3.3. In Vivo Experiments: Hypothyroidism Induction and Thyroxine Replacement
3.4. Western Blotting
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wiener, C.M. Harrison’s Principles of Internal Medicine Self-Assessment and Board Review, 17th ed.; McGraw-Hill, Medical: New York, NY, USA, 2008; p. 1166. [Google Scholar]
- Berne, R.M.; Koeppen, B.M. Berne & Levy Physiology, 6th ed.; Mosby/Elsevier: Philadelphia, PA, USA, 2010; pp. 721–723. [Google Scholar]
- Werner, S.C.; Ingbar, S.H.; Braverman, L.E.; Utiger, R.D. Werner & Ingbar’s the Thyroid: A Fundamental and Clinical Text, 9th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; p. 1166. [Google Scholar]
- Quesada, A.; Sainz, J.; Wangensteen, R.; Rodriguez-Gomez, I.; Vargas, F.; Osuna, A. Nitric oxide synthase activity in hyperthyroid and hypothyroid rats. Eur. J. Endocrinol. 2002, 147, 117–122. [Google Scholar]
- Chen, J.J.; Wang, S.W.; Chien, E.J.; Wang, P.S. Direct effect of propylthiouracil on progesterone release in rat granulosa cells. Br. J. Pharmacol. 2003, 139, 1564–1570. [Google Scholar]
- Chiao, Y.C.; Lee, H.Y.; Wang, S.W.; Hwang, J.J.; Chien, C.H.; Huang, S.W.; Lu, C.C.; Chen, J.J.; Tsai, S.C.; Wang, P.S. Regulation of thyroid hormones on the production of testosterone in rats. J. Cell Biochem. 1999, 73, 554–562. [Google Scholar] [CrossRef]
- Doong, M.L.; Wang, J.W.; Chung, S.C.; Liu, J.Y.; Hwang, C.; Hwang, C.Y.; Day, C.H.; Liu, Y.F.; Young, T.K.; Ho, L.L.; et al. Regulation of thyroid hormones in the secretion of insulin and gastric inhibitory polypeptide in male rats. Metabolism 1997, 46, 154–158. [Google Scholar] [CrossRef]
- Fernandez-Lamo, I.; Montero-Pedrazuela, A.; Delgado-Garcia, J.M.; Guadano-Ferraz, A.; Gruart, A. Effects of thyroid hormone replacement on associative learning and hippocampal synaptic plasticity in adult hypothyroid rats. Eur. J. Neurosci. 2009, 30, 679–692. [Google Scholar] [CrossRef] [Green Version]
- Shrader, R.E.; Keen, C.L.; Hurley, L.S.; Zeman, F.J. Hematologic and trace element alterations following chronic maternal ingestion of propylthiourea. Exp. Hematol. 1982, 10, 44–55. [Google Scholar]
- Chen, M.C.; Wang, S.W.; Kan, S.F.; Tsai, S.C.; Wu, Y.C.; Wang, P.S. Stimulatory effects of propylthiouracil on pregnenolone production through upregulation of steroidogenic acute regulatory protein expression in rat granulosa cells. Toxicol. Sci. 2010, 118, 667–674. [Google Scholar]
- Pu, H.F.; Lin, C.W.; Lee, H.W.; Wang, P.S. Effects of propylthiouracil on the production of aldosterone in rat zona glomerulosa cells. Adapt. Med. 2012, 4, 245–250. [Google Scholar] [CrossRef]
- Yo, P.L.; Lee, W.H.; Huang, W.J.; Wang, P.S. Adaptation of the secretion of gastric acid and gastric inhibitory peptide in response to propylthiouracil. Adapt. Med. 2011, 3, 106–111. [Google Scholar]
- Smith, R.G.; Cheng, K.; Schoen, W.R.; Pong, S.S.; Hickey, G.; Jacks, T.; Butler, B.; Chan, W.W.; Chaung, L.Y.; Judith, F.; et al. A nonpeptidyl growth hormone secretagogue. Science 1993, 260, 1640–1643. [Google Scholar]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Cheng, K.; Chan, W.W.; Butler, B.; Wei, L.; Schoen, W.R.; Wyvratt, M.J., Jr.; Fisher, M.H.; Smith, R.G. Stimulation of growth hormone release from rat primary pituitary cells by l-692,429, a novel non-peptidyl gh secretagogue. Endocrinology 1993, 132, 2729–2731. [Google Scholar]
- Briatore, L.; Andraghetti, G.; Cordera, R. Acute plasma glucose increase, but not early insulin response, regulates plasma ghrelin. Eur. J. Endocrinol. 2003, 149, 403–406. [Google Scholar] [CrossRef]
- Parker, B.A.; Doran, S.; Wishart, J.; Horowitz, M.; Chapman, I.M. Effects of small intestinal and gastric glucose administration on the suppression of plasma ghrelin concentrations in healthy older men and women. Clin. Endocrinol. 2005, 62, 539–546. [Google Scholar] [CrossRef]
- Sato, T.; Fukue, Y.; Teranishi, H.; Yoshida, Y.; Kojima, M. Molecular forms of hypothalamic ghrelin and its regulation by fasting and 2-deoxy-d-glucose administration. Endocrinology 2005, 146, 2510–2516. [Google Scholar] [CrossRef]
- Molina, P.E. Endocrine Physiology, 3rd ed.; McGraw-Hill Medical: New York, NY, USA, 2010; p. 303. [Google Scholar]
- Chang, Y.J.; Chen, C.M.; Chang, F.Y.; Wang, P.S. Radioimmunoassay for ghrelin: Evaluation of method and the effect of fasting in rats. Adapt. Med. 2010, 2, 108–112. [Google Scholar]
- Gannon, M.C.; Nuttall, F.Q. Effect of a high-protein diet on ghrelin, growth hormone, and insulin-like growth factor-i and binding proteins 1 and 3 in subjects with type 2 diabetes mellitus. Metabolism 2011, 60, 1300–1311. [Google Scholar] [CrossRef]
- Chang, Y.J.; Huang, W.J.; Lin, H.W.; Chang, L.L.; Chang, F.Y.; Wang, P.S. A radioimmunoassay for rat ghrelin: Evaluation of method and effects of nonylphenol on ghrelin secretion in force-fed young rats. Chin. J. Physiol. 2011, 54, 324–331. [Google Scholar]
- Romano, R.M.; Bargi-Souza, P.; Brunetto, E.L.; Goulart-Silva, F.; Avellar, M.C.; Oliveira, C.A.; Nunes, M.T. Hypothyroidism in adult male rats alters posttranscriptional mechanisms of luteinizing hormone biosynthesis. Thyroid 2013, 23, 497–505. [Google Scholar] [CrossRef]
- Dittrich, R.; Beckmann, M.W.; Oppelt, P.G.; Hoffmann, I.; Lotz, L.; Kuwert, T.; Mueller, A. Thyroid hormone receptors and reproduction. J. Reprod. Immunol. 2011, 90, 58–66. [Google Scholar] [CrossRef]
- Gimenez-Palop, O.; Gimenez-Perez, G.; Mauricio, D.; Berlanga, E.; Potau, N.; Vilardell, C.; Arroyo, J.; Gonzalez-Clemente, J.M.; Caixas, A. Circulating ghrelin in thyroid dysfunction is related to insulin resistance and not to hunger, food intake or anthropometric changes. Eur. J. Endocrinol. 2005, 153, 73–79. [Google Scholar] [CrossRef]
- Riis, A.L.; Hansen, T.K.; Moller, N.; Weeke, J.; Jorgensen, J.O. Hyperthyroidism is associated with suppressed circulating ghrelin levels. J. Clin. Endocrinol. Metab. 2003, 88, 853–857. [Google Scholar] [CrossRef]
- Caminos, J.E.; Seoane, L.M.; Tovar, S.A.; Casanueva, F.F.; Dieguez, C. Influence of thyroid status and growth hormone deficiency on ghrelin. Eur. J. Endocrinol. 2002, 147, 159–163. [Google Scholar] [CrossRef]
- Faix, J.D. Principles and pitfalls of free hormone measurements. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 631–645. [Google Scholar] [CrossRef]
- Dayan, C.M. Interpretation of thyroid function tests. Lancet 2001, 357, 619–624. [Google Scholar] [CrossRef]
- Ishii, S.; Kamegai, J.; Tamura, H.; Shimizu, T.; Sugihara, H.; Oikawa, S. Triiodothyronine (t3) stimulates food intake via enhanced hypothalamic amp-activated kinase activity. Regul. Pept. 2008, 151, 164–169. [Google Scholar] [CrossRef]
- Sarati, L.I.; Toblli, J.E.; Martinez, C.R.; Uceda, A.; Feldman, M.; Balaszczuk, A.M.; Fellet, A.L. Nitric oxide and aqp2 in hypothyroid rats: A link between aging and water homeostasis. Metabolism 2013, 62, 1287–1295. [Google Scholar] [CrossRef]
- Lawnicka, H.; Melen-Mucha, G.; Motylewska, E.; Mucha, S.; Stepien, H. Modulation of ghrelin axis influences the growth of colonic and prostatic cancer cells in vitro. Pharmacol. Rep. 2012, 64, 951–959. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Zhao, T.J.; Li, R.L.; Sherbet, D.P.; Liang, G.; Brown, M.S. Surviving starvation: Essential role of the ghrelin-growth hormone axis. Cold Spring Harb. Symp.Quant. Biol. 2011, 76, 121–127. [Google Scholar] [CrossRef]
- Wren, A.M.; Seal, L.J.; Cohen, M.A.; Brynes, A.E.; Frost, G.S.; Murphy, K.G.; Dhillo, W.S.; Ghatei, M.A.; Bloom, S.R. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 2001, 86, 5992–5995. [Google Scholar] [CrossRef]
- Kosowicz, J.; Baumann-Antczak, A.; Ruchala, M.; Gryczynska, M.; Gurgul, E.; Sowinski, J. Thyroid hormones affect plasma ghrelin and obestatin levels. Horm. Metab. Res. 2011, 43, 121–125. [Google Scholar] [CrossRef]
- Gjedde, S.; Vestergaard, E.T.; Gormsen, L.C.; Riis, A.L.; Rungby, J.; Moller, N.; Weeke, J.; Jorgensen, J.O. Serum ghrelin levels are increased in hypothyroid patients and become normalized by l-thyroxine treatment. J. Clin. Endocrinol. Metab. 2008, 93, 2277–2280. [Google Scholar] [CrossRef]
- Sawicka, B.; Bossowski, A.; Szalecki, M.; Wysoka, J.; Koput, A.; Zelazowska-Rutkowska, B.; Tobolczyk, J.; Rogowski, F.; Luba, M. Relationship between metabolic parameters and thyroid hormones and the level of gastric peptides in children with autoimmune thyroid diseases. J. Pediatr. Endocrinol. Metab. 2010, 23, 345–354. [Google Scholar]
- Evans, R.M.; Birnberg, N.C.; Rosenfeld, M.G. Glucocorticoid and thyroid hormones transcriptionally regulate growth hormone gene expression. Proc. Natl. Acad. Sci. USA 1982, 79, 7659–7663. [Google Scholar] [CrossRef]
- Cattini, P.A.; Anderson, T.R.; Baxter, J.D.; Mellon, P.; Eberhardt, N.L. The human growth hormone gene is negatively regulated by triiodothyronine when transfected into rat pituitary tumor cells. J. Biol. Chem. 1986, 261, 13367–13372. [Google Scholar]
- Silverman, B.L.; Kaplan, S.L.; Grumbach, M.M.; Miller, W.L. Hormonal regulation of growth hormone secretion and messenger ribonucleic acid accumulation in cultured bovine pituitary cells. Endocrinology 1988, 122, 1236–1241. [Google Scholar] [CrossRef]
- Boulanger, L.; Andersen, P.H.; Gaudreau, P. Development of a site-directed polyclonal antibody against the pituitary growth hormone-releasing hormone receptor and its use to estimate ghrh receptor concentration in normal and hypothyroid rats. Neuroendocrinology 1999, 70, 117–127. [Google Scholar] [CrossRef]
- Mulloy, A.L.; Smith, T.J.; Stachura, M.E. Comparative effects of thyroxine and/or retinoic acid treatment in vivo on growth hormone synthesis and release by pituitaries from thyroidectomized rats. Horm. Metab. Res. 1992, 24, 466–470. [Google Scholar] [CrossRef]
- Vottero, A.; Guzzetti, C.; Loche, S. New aspects of the physiology of the gh-igf-1 axis. Endocr. Dev. 2013, 24, 96–105. [Google Scholar] [CrossRef]
- Romero, G.S.; Stephan, D.A.; Sperling, M.A.; Menon, R.K. Distinct sexual dimorphism in the effect of hypothyroidism on the expression of the growth hormone receptor and growth hormone-binding protein gene in rat liver. Horm. Res. 1996, 45, 273–278. [Google Scholar] [CrossRef]
- Akin, F.; Yaylali, G.F.; Turgut, S.; Kaptanoglu, B. Growth hormone/insulin-like growth factor axis in patients with subclinical thyroid dysfunction. Growth Horm. IGF Res. 2009, 19, 252–255. [Google Scholar]
- Schmid, C.; Zwimpfer, C.; Brandle, M.; Krayenbuhl, P.A.; Zapf, J.; Wiesli, P. Effect of thyroxine replacement on serum igf-i, igfbp-3 and the acid-labile subunit in patients with hypothyroidism and hypopituitarism. Clin. Endocrinol. 2006, 65, 706–711. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics: A Biometrical Approach, 2nd ed.; McGraw-Hill: Auckland, Newzealand, 1980; p. 633. [Google Scholar]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chang, Y.-J.; Hwu, C.-M.; Yeh, C.-C.; Wang, P.S.; Wang, S.-W. Effects of Subacute Hypothyroidism on Metabolism and Growth-Related Molecules. Molecules 2014, 19, 11178-11195. https://doi.org/10.3390/molecules190811178
Chang Y-J, Hwu C-M, Yeh C-C, Wang PS, Wang S-W. Effects of Subacute Hypothyroidism on Metabolism and Growth-Related Molecules. Molecules. 2014; 19(8):11178-11195. https://doi.org/10.3390/molecules190811178
Chicago/Turabian StyleChang, Yen-Jui, Chii-Min Hwu, Chii-Chang Yeh, Paulus S. Wang, and Shyi-Wu Wang. 2014. "Effects of Subacute Hypothyroidism on Metabolism and Growth-Related Molecules" Molecules 19, no. 8: 11178-11195. https://doi.org/10.3390/molecules190811178
APA StyleChang, Y.-J., Hwu, C.-M., Yeh, C.-C., Wang, P. S., & Wang, S.-W. (2014). Effects of Subacute Hypothyroidism on Metabolism and Growth-Related Molecules. Molecules, 19(8), 11178-11195. https://doi.org/10.3390/molecules190811178



