Berberine, an Epiphany Against Cancer
Abstract
:1. Introduction
2. Berberine

3. Molecular Targets of Berberine
3.1. DNA
3.2. Cell Cycle
3.3. GADD (DNA Damage-Inducible Gene) 153
3.4. Cyclooxygenases (COX)
3.5. Mcl-1
3.6. Nucleophosmin/B23
3.7. Telomerase
3.8. Wnt
3.9. DAXX
3.10. AMPK
3.11. Enzymes Regulating Folate Cycle
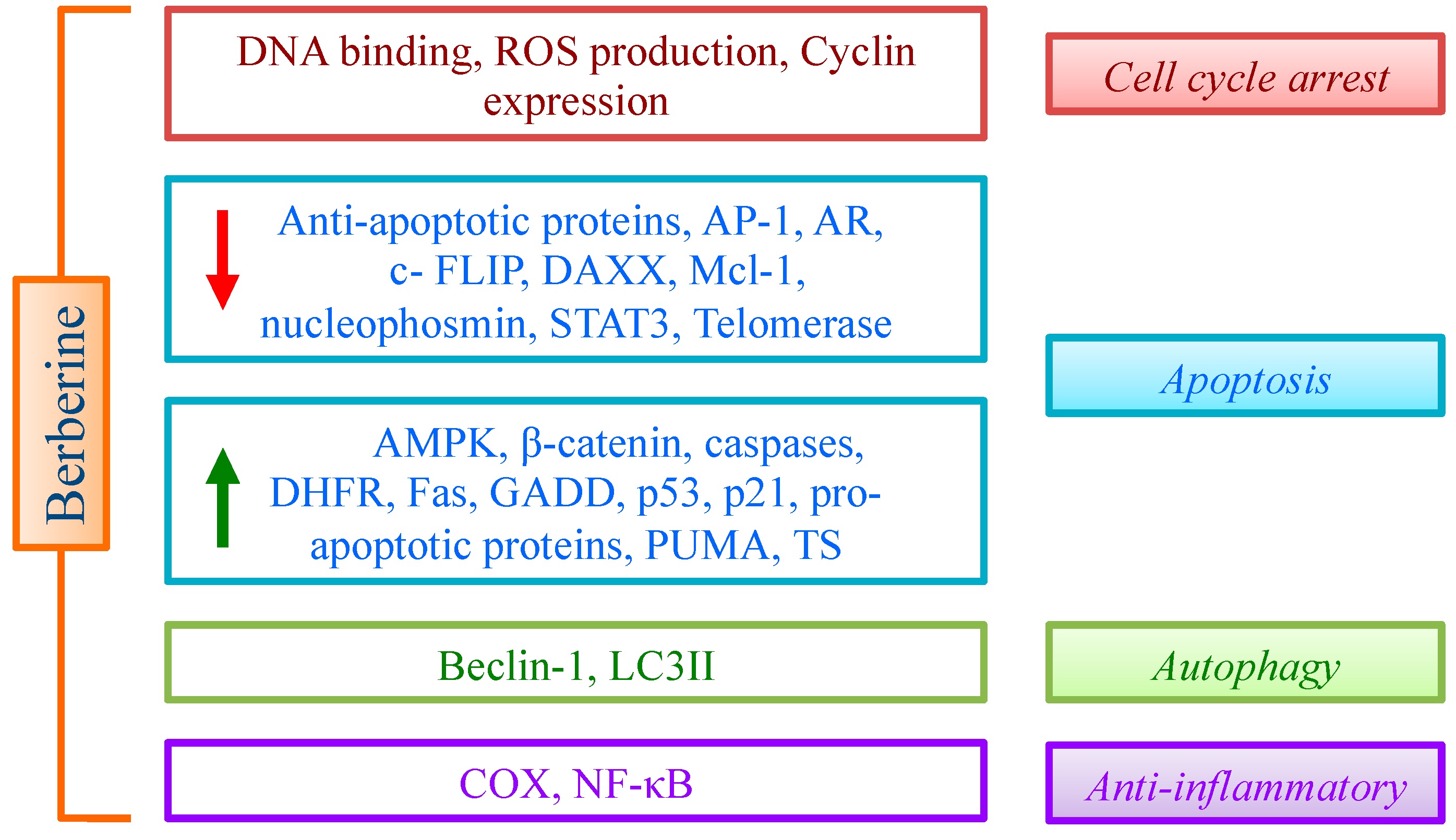
4. Berberine and Cancer
| Cell Line | Origin | Effect | Ref. |
|---|---|---|---|
| 8505C, TPC1 | Thyroid carcinoma | Cell cycle arrest | [28] |
| OVCAR-3, Skov-3 | Ovarian carcinoma | Cell cycle arrest | [29,63] |
| SCC-4, HSC-3 | Oral squamous carcinoma | Caspase activation; MMP disruption; Cytochrome c release; Cell cycle arrest; ROS production | [64,65] |
| SK-N-SH, SK-N-MCT98G | NeuroblastomaGlioblastoma | Caspase activation; PARP-1 cleavage | [66,67] |
| A375, Hs29 | Melanoma | COX-2 downregulation | [43] |
| HONE-1, NPC, C666-1 | Nasopharyngeal carcinoma | Caspase activation; PARP-1 cleavage; STAT3 inhibition; Mcl-1 downregulation | [46,47,68] |
| Panc-1 | Pancreatic cancer | TRAIL activation | [49] |
| A549, H1299 | Lung cancer | Caspase activation; MMP disruption; Bcl-2/Bcl-xL decrease; COX-2 downregulation; Cell cycle arrest | [30,42,69] |
| MCF-7, MDA-MB-231, MDA-MB-468, SK-BR-3 | Breast cancer | Caspase activation; PARP-1 cleavage; Cytochrome c release; Cell cycle arrest | [27,37,49,70,71,72] |
| HepG2 | Hepatoma | Caspase activation; PARP-1 cleavage; MMP disruption; Cytochrome c release; Bcl-2/Bcl-xL decrease | [73] |
| IMCE, HCT-116, SW480, SW620, SW613 | Colorectal cancer | Caspase activation; PARP-1 cleavage; ROS production; Cytochrome c release; Cell cycle arrest | [33,74,75,76] |
| LNCaP, PC-3, DU145, C4-2B | Prostate carcinoma | Caspase activation; PARP-1 cleavage; ROS production; MMP disruption; Cytochrome c release; Bcl-2/Bcl-xL decrease | [77,78,79] |
| A431 | Epidermoid carcinoma | Caspase activation; PARP-1 cleavage; MMP disruption; Bcl-2/Bcl-xL decrease | [80] |
| U937, HL-60 | Lymphoma, leukemia | Caspase activation; ROS production | [81,82,83] |
| SiHa, HeLa | Cervical cancer | Caspase activation; Telomerase downregulation | [84] |
4.1. Combined Use with Drugs and Radiation
4.2. Effect on Tumor Progression and Metastasis
4.3. Induction of Autophagy
5. BBR Derivatives for Anticancer Drug Discovery
6. Conclusions and New Perspectives
Acknowledgments
Author Contribution
Conflicts of Interest
References
- Guamán Ortiz, L.M.; Scovassi, A.I. Traditional medicine: An ancient remedy rediscovered. Biochem. Pharmacol. 2013, 2, 1. [Google Scholar]
- Kohler, J.C.; Baghdadi-Sabeti, G. Traditional medicines: Global situation, issues and challenges. The World Medicines Situation 2011, 3rd ed.; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Teiten, M.H.; Gaascht, F.; Dicato, M.; Diederich, M. Anticancer bioactivity of compounds from medicinal plants used in European medieval traditions. Biochem. Pharmacol. 2013, 86, 1239–1247. [Google Scholar] [CrossRef]
- Orlikova, B.; Legrand, N.; Panning, J.; Dicato, M.; Diederich, M. Anti-inflammatory and anticancer drugs from nature. Cancer Treat. Res. 2014, 159, 123–143. [Google Scholar]
- Wang, P.; Chen, Z. Traditional Chinese medicine ZHENG and Omics convergence: A systems approach to post-genomics medicine in a global world. OMICS 2013, 17, 451–459. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Y.F. Natural compounds as anticancer agents: Experimental evidence. World J. Exp. Med. 2012, 2, 45–57. [Google Scholar] [CrossRef]
- Chen, X.W.; Di, Y.M.; Zhang, J.; Zhou, Z.W.; Li, C.G.; Zhou, S.F. Interaction of herbal compounds with biological targets: A case study with berberine. Sci. World J. 2012, 2012, 708292. [Google Scholar]
- Tillhon, M.; Guamán Ortiz, L.M.; Lombardi, P.; Scovassi, A.I. Berberine: New perspectives for old remedies. Biochem. Pharmacol. 2012, 84, 1260–1267. [Google Scholar]
- Tang, Q.L.; Lai, M.L.; Zhong, Y.F.; Wang, A.M.; Su, J.K.; Zhang, M.Q. Antinociceptive effect of berberine on visceral hypersensitivity in rats. World J. Gastroenterol. 2013, 19, 4582–4589. [Google Scholar]
- Zhang, M.; Wang, C.M.; Li, J.; Meng, Z.J.; Wei, S.N.; Li, J.; Bucala, R.; Li, Y.L.; Chen, L. Berberine protects against palmitate-induced endothelial dysfunction: Involvements of upregulation of AMPK and eNOS and downregulation of NOX4. Mediators Inflamm. 2013, 2013, 260464. [Google Scholar]
- Heidarian, E.; Rafieian-Kopaei, M.; Khoshdel, A.; Bakhshesh, M. Metabolic effects of berberine on liver phosphatidate phosphohydrolase in rats fed on high lipogenic diet: An additional mechanism for the hypolipidemic effects of berberine. Asian Pac. J. Trop. Biomed. 2014, 4, S429–S435. [Google Scholar]
- Ansari, N.; Khodagholi, F. Natural products as promising drug candidates for the treatment of Alzheimer’s disease: Molecular mechanism aspect. Curr. Neuropharmacol. 2013, 11, 414–429. [Google Scholar] [CrossRef]
- Wu, Q.; Tang, Z.H.; Peng, J.; Liao, L.; Pan, L.H.; Wu, C.Y.; Jiang, Z.S.; Wang, G.X.; Liu, L.S. The dual behavior of PCSK9 in the regulation of apoptosis is crucial in Alzheimer’s disease progression. Biomed. Rep. 2014, 2, 167–171. [Google Scholar]
- Zhang, X.; Gu, L.; Li, J.; Shah, N.; He, J.; Yang, L.; Hu, Q.; Zhou, M. Degradation of MDM2 by the interaction between berberine and DAXX leads to potent apoptosis in MDM2-overexpressing cancer cells. Cancer Res. 2010, 70, 9895–9904. [Google Scholar]
- Liu, Q.; Jiang, H.; Liu, Z.; Wang, Y.; Zhao, M.; Hao, C.; Feng, S.; Guo, H.; Xu, B.; Yang, Q.; et al. Berberine radiosensitizes human esophageal cancer cells by downregulating homologous recombination repair protein RAD51. PLoS One 2011, 6, e23427. [Google Scholar] [CrossRef]
- Li, J.; Gu, L.; Zhang, H.; Liu, T.; Tian, D.; Zhou, M.; Zhou, S. Berberine represses DAXX gene transcription and induces cancer cell apoptosis. Lab. Investig. 2013, 93, 354–364. [Google Scholar]
- Wang, Y.; Kheir, M.M.; Chai, Y.; Hu, J.; Xing, D.; Lei, F.; Du, L. Comprehensive study in the inhibitory effect of berberine on gene transcription, including TATA box. PLoS One 2011, 6, e23495. [Google Scholar]
- Bhowmik, D.; Buzzetti, F.; Fiorillo, G.; Lombardi, P.; Suresh Kumar, G. Spectroscopic studies on the binding interaction of novel 13-phenylalkyl analogs of the natural alkaloid berberine to nucleic acid triplexes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 120, 257–264. [Google Scholar] [CrossRef]
- Krishnan, P.; Bastow, K. The 9-position in berberine analogs is an important determinant of DNA topoisomerase II inhibition. AntiCancer Drug Des. 2000, 15, 255–264. [Google Scholar]
- Qin, Y.; Pang, J.Y.; Chen, W.H.; Zhao, Z.Z.; Liu, L.; Jiang, Z.H. Inhibition of DNA topoisomerase I by natural and synthetic mono- and dimeric protoberberine alkaloids. Chem. Biodivers. 2007, 4, 481–487. [Google Scholar]
- Kim, S.A.; Kwon, Y.; Kim, J.H.; Muller, M.T.; Chung, I.K. Induction of topoisomerase II-mediated DNA cleavage by a protoberberine alkaloid, berberrubine. Biochemistry 1998, 37, 16316–16324. [Google Scholar] [CrossRef]
- Gatto, B.; Sanders, M.M.; Yu, C.; Wu, H.Y.; Makhey, D.; LaVoie, E.J.; Liu, L.F. Identification of topoisomerase I as the cytotoxic target of the protoberberine alkaloid coralyne. Cancer Res. 1996, 56, 2795–2800. [Google Scholar]
- Kang, M.R.; Chung, I.K. Down-regulation of DNA topoisomerase II alpha in human colorectal carcinoma cells resistant to a protoberberine alkaloid, berberrubine. Mol. Pharmacol. 2002, 61, 879–884. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yamashita, Y.; Fujii, N.; Takaboshi, K.; Kawakami, T.; Kawamura, M.; Mizukami, T.; Nakano, H. Inhibitors of DNA topoisomerase I and II isolated from the Coptis rhizomes. Planta Med. 1995, 61, 414–418. [Google Scholar]
- Pilch, D.S.; Yu, C.; Makhey, D.; LaVoie, E.J.; Srinivasan, A.R.; Olson, W.K.; Sauers, R.R.; Breslauer, K.J.; Geacintov, N.E.; Liu, L.F. Minor groove-directed and intercalative ligand-DNA interactions in the poisoning of human DNA topoisomerase I by protoberberine analogs. Biochemistry 1997, 36, 12542–12553. [Google Scholar] [CrossRef]
- Sanders, M.M.; Liu, A.A.; Li, T.K.; Wu, H.Y.; Desai, S.D.; Mao, Y.; Rubin, E.H.; LaVoie, E.J.; Makhey, D.; Liu, L.F. Selective cytotoxicity of topoisomerase-directed protoberberines against glioblastoma cells. Biochem. Pharmacol. 1998, 56, 1157–1166. [Google Scholar] [CrossRef]
- Kim, J.B.; Yu, J.H.; Ko, E.; Lee, K.W.; Song, A.K.; Park, S.Y.; Shin, I.; Han, W.; Noh, D.Y. The alkaloid berberine inhibits the growth of anoikis-resistant MCF-7 and MDA-MB-231 breast cancer cell lines by inducing cell cycle arrest. Phytomedicine 2010, 17, 436–440. [Google Scholar] [CrossRef]
- Park, K.S.; Kim, J.B.; Bae, J.; Park, S.Y.; Jee, H.G,; Lee, K.E.; Youn, Y.K. Berberine inhibited the growth of thyroid cancer cell lines 8505C and TPC1. Yonsei Med. J. 2012, 53, 346–351. [Google Scholar]
- Park, K.S.; Kim, J.B.; Lee, S.J.; Bae, J. Berberine-induced growth inhibition of epithelial ovarian carcinoma cell lines. J. Obstet. Gynaecol. Res. 2012, 38, 535–540. [Google Scholar] [CrossRef]
- James, M.A.; Fu, H.; Liu, Y.; Chen, D.R.; You, M. Dietary administration of berberine or Phellodendron amurense extract inhibits cell cycle progression and lung tumorigenesis. Mol. Carcinog. 2011, 50, 1–7. [Google Scholar] [CrossRef]
- Serafim, T.L.; Oliveira, P.J.; Sardao, V.A.; Perkins, E.; Parke, D.; Holy, J. Different concentrations of berberine result in distinct cellular localization patterns and cell cycle effects in a melanoma cell line. Cancer Chemother. Pharmacol. 2008, 61, 1007–1018. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Q.; Xu, B.; Wu, J.; Guo, C.; Zhu, F.; Yang, Q.; Gao, G.; Gong, Y.; Shao, C. Berberine induces p53-dependent cell cycle arrest and apoptosis of human osteosarcoma cells by inflicting DNA damage. Mutat. Res. 2009, 662, 75–83. [Google Scholar] [CrossRef]
- Guamán Ortiz, L.M.; Tillhon, M.; Parks, M.; Dutto, I.; Prosperi, E.; Savio, M.; Arcamone, A.G.; Buzzetti, F.; Lombardi, P.; Scovassi, A.I. Multiple effects of berberine derivatives on colon cancer cells. BioMed. Res. Int. 2014, 2014, 924585. [Google Scholar]
- Thirupurasundari, C.J.; Padmini, R.; Devaraj, S.N. Effect of berberine on the antioxidant status, ultrastructural modifications and protein bound carbohydrates in azoxymethane-induced colon cancer in rats. Chem. Biol. Interact. 2009, 177, 190–195. [Google Scholar] [CrossRef]
- Aggarwal, B.; Prasad, S.; Sung, B.; Krishnan, S.; Guha, S. Prevention and treatment of colorectal cancer by natural agents from mother nature. Curr. Colorectal Cancer Rep. 2013, 9, 37–56. [Google Scholar] [CrossRef]
- Yip, N.K.; Ho, W.S. Berberine induces apoptosis via the mitochondrial pathway in liver cancer cells. Oncol. Rep. 2013, 30, 1107–1112. [Google Scholar]
- Pierpaoli, E.; Arcamone, A.G.; Buzzetti, F.; Lombardi, P.; Salvatore, C.; Provinciali, M. Antitumor effect of novel berberine derivatives in breast cancer cells. Biofactors 2013, 39, 672–679. [Google Scholar]
- Lin, J.P.; Yang, J.S.; Chang, N.W.; Chiu, T.H.; Su, C.C.; Lu, K.W.; Ho, Y.T.; Yeh, C.C.; Yang, M.D.; Lin, H.J.; et al. GADD153 mediates berberine-induced apoptosis in human cervical cancer Ca Ski cells. Anticancer Res. 2007, 27, 3379–3386. [Google Scholar]
- Yu, W.; Sheng, M.; Xu, R.; Yu, J.; Cui, K.; Tong, J.; Shi, L.; Ren, H.; Du, H. Berberine protects human renal proximal tubular cells from hypoxia/reoxygenation injury via inhibiting endoplasmic reticulum and mitochondrial stress pathways. J. Transl. Med. 2013, 11, 24. [Google Scholar]
- Dai, D.Z. CPU86017: A novel Class III antiarrhythmic agent with multiple actions at ion channels. Cardiovasc. Drug Rev. 2006, 24, 101–115. [Google Scholar] [CrossRef]
- Liu, G.L.; Yu, F.; Dai, D.Z.; Zhang, G.L.; Zhang, C.; Dai, Y. Endoplasmic reticulum stress mediating downregulated StAR and 3-beta-HSD and low plasma testosterone caused by hypoxia is attenuated by CPU86017-RS and nifedipine. J. Biomed. Sci. 2012, 19, 4. [Google Scholar] [CrossRef]
- Fu, L.; Chen, W.; Guo, W.; Wang, J.; Tian, Y.; Shi, D.; Zhang, X.; Qiu, H.; Xiao, X.; Kang, T.; et al. Berberine targets AP-2/hTERT, NF-κB/COX-2, HIF-1α/VEGF and cytochrome-c/caspase signaling to suppress human cancer cell growth. PLoS One 2013, 8, e69240. [Google Scholar]
- Singh, T.; Vaid, M.; Katiyar, N.; Sharma, S.; Katiyar, S.K. Berberine, an isoquinoline alkaloid, inhibits melanoma cancer cell migration by reducing the expressions of cyclooxygenase-2, prostaglandin E2 and prostaglandin E2 receptors. Carcinogenesis 2011, 32, 86–92. [Google Scholar] [CrossRef]
- Ma, X.; Jiang, Y.; Wu, A.; Chen, X.; Pi, R.; Liu, M.; Liu, Y. Berberine attenuates experimental autoimmune encephalomyelitis in C57 BL/6 mice. PLoS One 2010, 5, e13489. [Google Scholar]
- Sugio, A.; Iwasaki, M.; Habata, S.; Mariya, T.; Suzuki, M.; Osogami, H.; Tamate, M.; Tanaka, R.; Saito, T. BAG3 upregulates Mcl-1 through downregulation of miR-29b to induce anticancer drug resistance in ovarian cancer. Gynecol. Oncol. 2014. [Google Scholar] [CrossRef]
- Tsang, C.M.; Cheung, Y.C.; Lui, V.W.; Yip, Y.L.; Zhang, G.; Lin, V.W.; Cheung, K.C.; Feng, Y.; Tsao, S.W. Berberine suppresses tumorigenicity and growth of nasopharyngeal carcinoma cells by inhibiting STAT3 activation induced by tumor associated fibroblasts. BMC Cancer 2013, 13, 619. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.L.; Chi, C.W.; Liu, T.Y. Modulation of apoptosis by berberine through inhibition of cyclooxygenase-2 and Mcl-1 expression in oral cancer cells. In Vivo 2005, 19, 247–252. [Google Scholar]
- Refaat, A.; Abd-Rabou, A.; Reda, A. TRAIL combinations: The new “trail” for cancer therapy. Oncol. Lett. 2014, 7, 1327–1332. [Google Scholar]
- Refaat, A.; Abdelhamed, S.; Yagita, H.; Inoue, H.; Yokoyama, S.; Hayakawa, Y.; Saiki, I. Berberine enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast cancer. Oncol. Lett. 2013, 6, 840–844. [Google Scholar]
- Colombo, E.; Alcalay, M.; Pelicci, P.G. Nucleophosmin and its complex network: A possible therapeutic target in hematological diseases. Oncogene 2011, 30, 2595–2609. [Google Scholar] [CrossRef]
- Wu, H.L.; Hsu, C.Y.; Liu, WH.; Yung, B.Y. Berberine-induced apoptosis of human leukemia HL-60 cells is associated with down-regulation of nucleophosmin/B23 and telomerase activity. Int. J. Cancer 1999, 81, 923–929. [Google Scholar]
- Bernardes de Jesus, B.; Blasco, M.A. Telomerase at the intersection of cancer and aging. Trends Genet. 2013, 29, 513–520. [Google Scholar] [CrossRef]
- Günes, C.; Rudolph, K.L. The role of telomeres in stem cells and cancer. Cell 2013, 152, 390–393. [Google Scholar] [CrossRef]
- Bazzicalupi, C.; Ferraroni, M.; Bilia, A.R.; Scheggi, F.; Gratteri, P. The crystal structure of human telomeric DNA complexed with berberine: An interesting case of stacked ligand to G-tetrad ratio higher than 1:1. Nucleic Acids Res. 2013, 41, 632–638. [Google Scholar] [CrossRef]
- Ji, X.; Sun, H.; Zhou, H.; Xiang, J.; Tang, Y.; Zhao, C. The interaction of telomeric DNA and C-myc22 G-quadruplex with 11 natural alkaloids. Nucleic Acid Ther. 2012, 22, 127–136. [Google Scholar]
- Albring, K.F.; Weidemüller, J.; Mittag, S.; Weiske, J.; Friedrich, K.; Geroni, M.C.; Lombardi, P.; Huber, O. Berberine acts as a natural inhibitor of Wnt/β-catenin signaling-Identification of more active 13-arylalkyl derivatives. Biofactors 2013, 39, 652–662. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, Z.; Ratnam, M.; Dou, Q.P. The interplay of AMP-activated protein kinase and androgen receptor in prostate cancer cells. J. Cell. Physiol. 2014, 229, 688–695. [Google Scholar] [CrossRef]
- Jeong, K.J.; Kim, G.W.; Chung, S.H. AMP-activated protein kinase: An emerging target for ginseng. J. Ginseng Res. 2014, 38, 83–88. [Google Scholar] [CrossRef]
- Carreras, C.W.; Santi, D.V. The catalytic mechanism and structure of thymidylate synthase. Annu. Rev. Biochem. 1995, 64, 721–762. [Google Scholar]
- Costi, M.P.; Ferrari, S. Update on antifolate drugs targets. Curr. Drug Targets 2001, 2, 135–166. [Google Scholar] [CrossRef]
- Scanlon, K.J.; Kashani-Sabet, M. Elevated expression of thymidylate synthase cycle genes in cisplatin-resistant human ovarian carcinoma A2780 cells. Proc. Natl. Acad. Sci. USA 1988, 85, 650–653. [Google Scholar] [CrossRef]
- Scanlon, K.J.; Wang, W.Z.; Han, H. Cyclosporin A suppresses cisplatin-induced oncogene expression in human cancer cells. Cancer Treat. Rev. 1990, 17, 27–35. [Google Scholar] [CrossRef]
- Marverti, G.; Ligabue, A.; Lombardi, P.; Ferrari, S.; Monti, M.G.; Frassineti, C.; Costi, M.P. Modulation of the expression of folate cycle enzymes and polyamine metabolism by berberine in cisplatin-sensitive and -resistant human ovarian cancer cells. Int. J. Oncol. 2013, 43, 1269–1280. [Google Scholar]
- Lin, C.C.; Yang, J.S.; Chen, J.T.; Fan, S.; Yu, F.S.; Yang, J.L.; Lu, C.C.; Kao, M.C.; Huang, A.C.; Lu, H.F.; et al. Berberine induces apoptosis in human HSC-3 oral cancer cells via simultaneous activation of the death receptor-mediated and mitochondrial pathway. Anticancer Res. 2007, 27, 3371–3378. [Google Scholar]
- Ho, Y.T.; Lu, C.C.; Yang, J.S.; Chiang, J.H.; Li, T.C.; Ip, S.W.; Hsia, T.C.; Liao, C.L.; Lin, J.G.; Wood, W.G.; et al. Berberine induced apoptosis via promoting the expression of caspase-8, -9 and -3, apoptosis-inducing factor and endonuclease G in SCC-4 human tongue squamous carcinoma cancer cells. Anticancer Res. 2009, 29, 4063–4070. [Google Scholar]
- Choi, M.S.; Yuk, D.Y.; Oh, J.H.; Jung, H.Y.; Han, S.B.; Moon, D.C.; Hong, J.T. Berberine inhibits human neuroblastoma cell growth through induction of p53-dependent apoptosis. Anticancer Res. 2008, 28, 3777–3784. [Google Scholar]
- Eom, K.S.; Hong, J.M.; Youn, M.J.; So, H.S.; Park, R.; Kim, J.M.; Kim, T.Y. Berberine induces G1 arrest and apoptosis in human glioblastoma T98G cells through mitochondrial/caspases pathway. Biol. Pharm. Bull. 2008, 31, 558–562. [Google Scholar]
- Tsang, C.M.; Lau, E.P.; Di, K.; Cheung, P.Y.; Hau, P.M.; Ching, Y.P.; Wong, Y.C.; Cheung, A.L.; Wan, T.S.; Tong, Y.; et al. Berberine inhibits Rho GTPases and cell migration at low doses but induces G2 arrest and apoptosis at high doses in human cancer cells. Int. J. Mol. Med. 2009, 24, 131–138. [Google Scholar]
- Katiyar, S.K.; Meeran, S.M.; Katiyar, N.; Akhtar, S. p53 Cooperates berberine- induced growth inhibition and apoptosis of non-small cell human lung cancer cells in vitro and tumor xenograft growth in vivo. Mol. Carcinog. 2009, 48, 24–37. [Google Scholar]
- Patil, J.B.; Kim, J.; Jayaprakasha, G.K. Berberine induces apoptosis in breast cancer cells (MCF-7) through mitochondrial-dependent pathway. Eur. J. Pharmacol. 2010, 645, 70–78. [Google Scholar] [CrossRef]
- Kuo, H.P.; Chuang, T.C.; Yeh, M.H.; Hsu, S.C.; Way, T.D.; Chen, P.Y.; Wang, S.S.; Chang, Y.H.; Kao, M.C.; Liu, J.Y. Growth suppression of HER2-overexpressing breast cancer cells by berberine via modulation of the HER2/PI3K/Akt signaling pathway. J. Agric. Food Chem. 2011, 59, 8216–8224. [Google Scholar]
- Kuo, H.P.; Chuang, T.C.; Tsai, S.C.; Tseng, H.H.; Hsu, S.C.; Chen, Y.C.; Kuo, C.L.; Kuo, Y.H.; Liu, J.Y.; Kao, M.C. Berberine, an isoquinoline alkaloid, inhibits the metastatic potential of breast cancer cells via Akt pathway modulation. J. Agric. Food Chem. 2012, 60, 9649–9658. [Google Scholar]
- Hwang, J.M.; Kuo, H.C.; Tseng, T.H.; Liu, J.Y.; Chu, C.Y. Berberine induces apoptosis through a mitochondria/caspases pathway in human hepatoma cells. Arch. Toxicol. 2006, 80, 62–73. [Google Scholar] [CrossRef]
- Wang, L.; Liu, L.; Shi, Y.; Cao, H.; Chaturvedi, R.; Calcutt, M.W.; Hu, T.; Ren, X.; Wilson, K.T.; Polk, D.B.; et al. Berberine induces caspase-independent cell death in colon tumor cells through activation of apoptosis-inducing factor. PLoS One 2012, 7, e36418. [Google Scholar]
- Piyanuch, R.; Sukhthankar, M.; Wandee, G.; Baek, S.J. Berberine, a natural iso-quinoline alkaloid, induces NAG-1 and ATF3 expression in human colorectal cancer cells. Cancer Lett. 2007, 258, 230–240. [Google Scholar] [CrossRef]
- Hsu, W.H.; Hsieh, Y.S.; Kuo, H.C.; Teng, C.Y.; Huang, H.I.; Wang, C.J.; Yang, S.F.; Liou, Y.S.; Kuo, W.H. Berberine induces apoptosis in SW620 human colonic carcinoma cells through generation of reactive oxygen species and activation of JNK/p38 MAPK and FasL. Arch. Toxicol. 2007, 81, 719–728. [Google Scholar] [CrossRef]
- Choi, M.S.; Oh, J.H.; Kim, S.M.; Jung, H.Y.; Yoo, H.S.; Lee, Y.M.; Moon, D.C.; Han, S.B.; Hong, J.T. Berberine inhibits p53-dependent cell growth through induction of apoptosis of prostate cancer cells. Int. J. Oncol. 2009, 34, 1221–1230. [Google Scholar]
- Meeran, S.M.; Katiyar, S.; Katiyar, S.K. Berberine-induced apoptosis in human prostate cancer cells is initiated by reactive oxygen species generation. Toxicol. Appl. Pharmacol. 2008, 229, 33–43. [Google Scholar] [CrossRef]
- Mantena, S.K.; Sharma, S.D.; Katiyar, S.K. Berberine, a natural product, induces G1-phase cell cycle arrest and caspase-3-dependent apoptosis in human prostate carcinoma cells. Mol. Cancer Ther. 2006, 5, 296–308. [Google Scholar]
- Mantena, S.K.; Sharma, S.D.; Katiyar, S.K. Berberine inhibits growth, induces G1 arrest and apoptosis in human epidermoid carcinoma A431 cells by regulating Cdki-Cdk-cyclin cascade, disruption of mitochondrial membrane potential and cleavage of caspase 3 and PARP. Carcinogenesis 2006, 27, 2018–2027. [Google Scholar] [CrossRef]
- Letasiova, S.; Jantova, S.; Cipak, L.; Muckova, M. Berberine-antiproliferative activity in vitro and induction of apoptosis/necrosis of the U937 and B16 cells. Cancer Lett. 2006, 239, 254–262. [Google Scholar]
- Jantova, S.; Cipak, L.; Letasiova, S. Berberine induces apoptosis through a mitochondrial/caspase pathway in human promonocytic U937 cells. Toxicol. In Vitro 2007, 21, 25–31. [Google Scholar] [CrossRef]
- Lin, C.C.; Kao, S.T.; Chen, G.W.; Ho, H.C.; Chung, J.G. Apoptosis of human leukemia HL-60 cells and murine leukemia WEHI-3 cells induced by berberine through the activation of caspase-3. Anticancer Res. 2006, 26, 227–242. [Google Scholar]
- Mahata, S.; Bharti, A.C.; Shukla, S.; Tyagi, A.; Husain, S.A.; Das, B.C. Berberine modulates AP-1 activity to suppress HPV transcription and downstream signaling to induce growth arrest and apoptosis in cervical cancer cells. Mol. Cancer 2011, 10, 39. [Google Scholar]
- Pandey, M.K.; Sung, B.; Kunnumakkara, A.B.; Sethi, G.; Chaturvedi, M.M.; Aggarwal, B.B. Berberine modifies cysteine 179 of IκBαkinase, suppresses nuclear factor-κB-regulated antiapoptotic gene products, and potentiates apoptosis. Cancer Res. 2008, 68, 5370–5379. [Google Scholar]
- Fukuda, K.; Hibiya, Y.; Mutoh, M.; Koshiji, M.; Akao, S.; Fujiwara, H. Inhibition of activator protein 1 activity by berberine in human hepatoma cells. Planta Med. 1999, 65, 381–383. [Google Scholar] [CrossRef]
- Kuo, C.L.; Chi, C.W.; Liu, T.Y. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004, 203, 127–137. [Google Scholar]
- Kim, S.; Choi, J.H.; Kim, J.B.; Nam, S.J.; Yang, J.H.; Kim, J.H.; Lee, J.E. Berberine suppresses TNF-α-induced MMP-9 and cell invasion through inhibition of AP-1 activity in MDA-MB-231 human breast cancer cells. Molecules 2008, 13, 2975–2985. [Google Scholar] [CrossRef]
- Fukuda, K.; Hibiya, Y.; Mutoh, M.; Koshiji, M.; Akao, S.; Fujiwara, H. Inhibition by berberine of cyclooxygenase-2 transcriptional activity in human colon cancer cells. J. Ethnopharmacol. 1999, 66, 227–233. [Google Scholar] [CrossRef]
- Lin, T.H.; Kuo, H.C.; Chou, F.P.; Lu, F.J. Berberine enhances inhibition of glioma tumor cell migration and invasiveness mediated by arsenic trioxide. BMC Cancer 2008, 8, 58. [Google Scholar]
- Kim, D.W.; Ahan, S.H.; Kim, T.Y. Enhancement of arsenic trioxide (As2O3)-mediated apoptosis using berberine in human neuroblastoma SH-SY5Y cells. J. Korean Neurosurg. Soc. 2007, 42, 392–399. [Google Scholar] [CrossRef]
- Youn, M.J.; So, H.S.; Cho, H.J.; Kim, H.J.; Kim, Y.; Lee, J.H.; Sohn, J.S.; Kim, Y.K.; Chung, S.Y.; Park, R. Berberine, a natural product, combined with cisplatin enhanced apoptosis through a mitochondria/caspase-mediated pathway in HeLa cells. Biol. Pharm. Bull. 2008, 31, 789–795. [Google Scholar]
- Wang, X.N.; Han, X.; Xu, L.N.; Yin, L.H.; Xu, Y.W.; Qi, Y.; Peng, J.Y. Enhancement of apoptosis of human hepatocellular carcinoma SMMC-7721 cells through synergy of berberine and evodiamine. Phytomedicine 2008, 15, 1062–1068. [Google Scholar] [CrossRef]
- Liu, J.; He, C.; Zhou, K.; Wang, J.; Kang, J.X. Coptis extracts enhance the anticancer effect of estrogen receptor antagonists on human breast cancer cells. Biochem. Biophys. Res. Commun. 2009, 378, 174–178. [Google Scholar] [CrossRef]
- Yu, M.; Tong, X.; Qi, B.; Qu, H.; Dong, S.; Yu, B.; Zhang, N.; Tang, N.; Wang, L.; Zhang, C. Berberine enhances chemosensitivity to irinotecan in colon cancer via inhibition of NF-κB. Mol. Med. Rep. 2014, 9, 249–254. [Google Scholar]
- Wang, L.; Wei, D.; Han, X.; Zhang, W.; Fan, C.; Zhang, J.; Mo, C.; Yang, M.; Li, J.; Wang, Z.; et al. The combinational effect of vincristine and BBR on growth inhibition and apoptosis induction in hepatoma cells. J. Cell. Biochem. 2014, 115, 721–730. [Google Scholar] [CrossRef]
- Peng, P.L.; Kuo, W.H.; Tseng, H.C.; Chou, F.P. Synergistic tumor-killing effect of radiation and berberine combined treatment in lung cancer: the contribution of autophagic cell death. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 529–542. [Google Scholar]
- Hur, J.M.; Hyun, M.S.; Lim, S.Y.; Lee, W.Y.; Kim, D. The combination of berberine and irradiation enhances anti-cancer effects via activation of p38 MAPK pathway and ROS generation in human hepatoma cells. J. Cell. Biochem. 2009, 107, 955–964. [Google Scholar] [CrossRef]
- Yang, X.; Yang, B.; Cai, J.; Zhang, C.; Zhang, Q.; Xu, L.; Qin, Q.; Zhu, H.; Ma, J.; Tao, G.; et al. Berberine enhances radiosensitivity of esophageal squamous cancer by targeting HIF-1α in vitro and in vivo. Cancer Biol. Ther. 2013, 14, 1068–1073. [Google Scholar] [CrossRef]
- Qi, H.W.; Xin, L.Y.; Xu, X.; Ji, X.X.; Fan, L.H. Epithelial-to-mesenchymal transition markers to predict response of Berberine in suppressing lung cancer invasion and metastasis. J. Transl. Med. 2014, 12, 22. [Google Scholar] [CrossRef]
- Tang, F.; Wang, D.; Duan, C.; Huang, D.; Wu, Y.; Chen, Y.; Wang, W.; Xie, C.; Meng, J.; Wang, L.; et al. Berberine inhibits metastasis of nasopharyngeal carcinoma 5–8F cells by targeting Rho kinase-mediated Ezrin phosphorylation at threonine 567. J. Biol. Chem. 2009, 284, 27456–27466. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, M.J.; Kim, E.J.; Yang, Y.; Lee, M.S.; Lim, J.S. Berberine-induced AMPK activation inhibits the metastatic potential of melanoma cells via reduction of ERK activity and COX-2 protein expression. Biochem. Pharmacol. 2012, 83, 385–394. [Google Scholar]
- Hamsa, T.P.; Kuttan, G. Berberine inhibits pulmonary metastasis through down-regulation of MMP in metastatic B16F-10 melanoma cells. Phytother. Res. 2012, 26, 568–578. [Google Scholar] [CrossRef]
- Park, J.J.; Seo, S.M.; Kim, E.J.; Lee, Y.J.; Ko, Y.G.; Ha, J.; Lee, M. Berberine inhibits human colon cancer cell migration via AMP-activated protein kinase-mediated downregulation of integrin β1 signaling. Biochem. Biophys. Res. Commun. 2012, 426, 461–467. [Google Scholar] [CrossRef]
- Wu, C.M.; Li, T.M.; Tan, T.W.; Fong, Y.C.; Tang, C.H. Berberine reduces the metastasis of chondrosarcoma by modulating the αvβ3 integrin and the PKCδ, c-Src, and AP-1 signaling pathways. Evid. Based Complement. Altern. Med. 2013. [Google Scholar] [CrossRef]
- Peng, P.L.; Hsieh, Y.S.; Wang, C.J.; Hsu, J.L.; Chou, F.P. Inhibitory effect of berberine on the invasion of human lung cancer cells via decreased productions of urokinase-plasminogen activator and matrix metalloproteinase-2. Toxicol. Appl. Pharmacol. 2006, 214, 8–15. [Google Scholar] [CrossRef]
- Aredia, F.; Guamán Ortiz, L.M.; Giansanti, V.; Scovassi, A.I. Autophagy and cancer. Cells 2012, 1, 520–534. [Google Scholar] [CrossRef]
- Wu, W.K.; Coffelt, S.B.; Cho, C.H.; Wang, X.J.; Lee, C.W.; Chan, F.K.; Yu, J.; Sung, J.J. The autophagic paradox in cancer therapy. Oncogene 2012, 31, 939–953. [Google Scholar]
- Gewirtz, D.A. The four faces of autophagy: Implications for cancer therapy. Cancer Res. 2014, 74, 647–651. [Google Scholar]
- Eisenberg-Lerner, A.; Kimchi, A. The paradox of autophagy and its implication in cancer etiology and therapy. Apoptosis 2009, 14, 376–391. [Google Scholar] [CrossRef]
- Mariño, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar]
- Loos, B.; Engelbrecht, A.M.; Lockshin, R.A.; Klionsky, D.J.; Zakeri, Z. The variability of autophagy and cell death susceptibility: Unanswered questions. Autophagy 2013, 9, 1270–1285. [Google Scholar]
- Giansanti, V.; Tillhon, M.; Mazzini, G.; Prosperi, E.; Lombardi, P.; Scovassi, A.I. Killing of tumor cells: A drama in two acts. Biochem. Pharmacol. 2011, 82, 1304–1310. [Google Scholar] [CrossRef]
- Tan, W.; Li, Y.; Chen, M.; Wang, Y. Berberine hydrochloride: Anticancer activity and nanoparticulate delivery system. Int. J. Nanomed. 2011, 6, 1773–1777. [Google Scholar]
- Hou, Q.; Tang, X.; Liu, H.; Tang, J.; Yang, Y.; Jing, X.; Xiao, Q.; Wang, W.; Gou, X.; Wang, Z. Berberine induces cell death in human hepatoma cells in vitro by downregulating CD147. Cancer Sci. 2011, 102, 1287–1292. [Google Scholar] [CrossRef]
- Giansanti, V.; Torriglia, A.; Scovassi, A.I. Conversation between apoptosis and autophagy: “Is it your turn or mine?”. Apoptosis 2011, 16, 321–333. [Google Scholar]
- Lenka, G.; Dostal, J.; Radek, M. Quaternary protoberberine alkaloids. Phytochemistry 2007, 68, 150–175. [Google Scholar] [CrossRef]
- Nechepurenko, I.V.; Salakhutdinov, N.F.; Tolstikov, G.A. Berberine: Chemistry and biological activity. Chem. Sustain. Dev. 2010, 18, 1–23. [Google Scholar]
- Singh, I.P.; Mahajan, S. Berberine and its derivatives: A patent review (2009–2012). Expert Opin. Ther. Pat. 2013, 23, 215–231. [Google Scholar] [CrossRef]
- Waters, M.L. Aromatic interactions in model systems. Curr. Opin. Chem. Biol. 2002, 6, 736–741. [Google Scholar]
- Riley, K.E.; Hobza, P. On the importance and origin of aromatic interactions in chemistry and biodisciplines. Acc. Chem. Res. 2013, 46, 927–936. [Google Scholar] [CrossRef]
- Bhowmik, D.; Hossain, M.; Buzzetti, F.; D’Auria, R.; Lombardi, P.; Kumar, G.S. Biophysical studies on the effect of the 13 position substitution of the anticancer alkaloid berberine on its DNA binding. J. Phys. Chem. B 2012, 116, 2314–2324. [Google Scholar]
- Bhowmik, D.; Buzzetti, F.; Fiorillo, G.; Orzi, F.; Syeda, T.M.; Lombardi, P.; Kumar, G.S. Synthesis of new 13-diphenylalkyl analogs of berberine and elucidation of their base pair specificity and energetics of DNA binding. Med. Chem. Commun. 2014, 5, 226–231. [Google Scholar]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 2, 281–297. [Google Scholar]
- Lo, T.F.; Tsai, W.C.; Chen, S.T. MicroRNA-21–3p, a berberine-induced miRNA, directly down-regulates human methionine adenosyltransferases 2A and 2B and inhibits hepatoma cell growth. PLoS One 2013, 8, e75628. [Google Scholar]
- Hu, H.Y.; Li, K.P.; Wang, X.J.; Liu, Y.; Lu, Z.G.; Dong, R.H.; Guo, H.B.; Zhang, M.X. Set9, NF-κB, and microRNA-21 mediate berberine-induced apoptosis of human multiple myeloma cells. Acta Pharmacol. Sin. 2013, 34, 157–166. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Y.; Shen, H.; Xu, W.; Li, H. Berberine sensitizes ovarian cancer cells to cisplatin through miR-21/PDCD4 axis. Acta Biochim. Biophys. Sin. 2013, 45, 756–762. [Google Scholar]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Guamán Ortiz, L.M.; Lombardi, P.; Tillhon, M.; Scovassi, A.I. Berberine, an Epiphany Against Cancer. Molecules 2014, 19, 12349-12367. https://doi.org/10.3390/molecules190812349
Guamán Ortiz LM, Lombardi P, Tillhon M, Scovassi AI. Berberine, an Epiphany Against Cancer. Molecules. 2014; 19(8):12349-12367. https://doi.org/10.3390/molecules190812349
Chicago/Turabian StyleGuamán Ortiz, Luis Miguel, Paolo Lombardi, Micol Tillhon, and Anna Ivana Scovassi. 2014. "Berberine, an Epiphany Against Cancer" Molecules 19, no. 8: 12349-12367. https://doi.org/10.3390/molecules190812349
APA StyleGuamán Ortiz, L. M., Lombardi, P., Tillhon, M., & Scovassi, A. I. (2014). Berberine, an Epiphany Against Cancer. Molecules, 19(8), 12349-12367. https://doi.org/10.3390/molecules190812349





