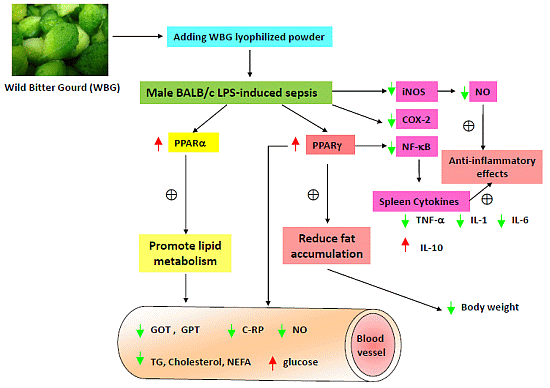
Anti-Inflammatory Effect of Momordica Charantia in Sepsis Mice
Abstract
:1. Introduction
2. Results and Discussion
| Group | n | Body Weight | Food Intake | |
|---|---|---|---|---|
| Initial BW (g) | Final BW (g) | (g/day) | ||
| N | 12 | 22.6 ± 2.0 a | 25.8 ± 1.7 b | 3.8 ± 0.2 a |
| S | 12 | 23.0 ± 1.5 a | 27.0 ± 1.3 a | 3.8 ± 0.3 a |
| L | 12 | 23.3 ± 1.0 a | 23.5 ± 0.9 c | 3.9 ± 0.3 a |
| M | 12 | 22.8 ± 1.3 a | 22.8 ± 0.5 c | 3.9 ± 0.3 a |
| H | 12 | 23.6 ± 1.3 a | 19.8 ± 1.5 d | 3.9 ± 0.6 a |
| P | 12 | 23.0 ± 0.7 a | 25.2 ± 0.5 a | 3.8 ± 0.2 a |
| Group | n | Triglyceride (mg/dL) | Cholesterol (mg/dL) | NEFA (mmol/L) | Glucose (mg/dL) |
|---|---|---|---|---|---|
| N | 12 | 186.8 ± 11.7 a | 194.7 ± 20.3 c | 0.64 ± 0.03 d | 102.6 ± 4.8 a |
| S | 12 | 224.2 ± 10.9 bc | 227.1 ± 36.0 a | 0.95 ± 0.05 a | 50.1 ± 5.2 c |
| L | 12 | 196.0 ± 6.6 c | 222.9 ± 15.5 b | 0.92 ± 0.01 a | 50.6 ± 4.9 c |
| M | 12 | 197.3 ± 23.9 c | 222.1 ± 24.2 a | 0.85 ± 0.01 a | 72.7 ± 16.4 b |
| H | 12 | 188.7 ± 21.4 d | 207.1 ± 13.8 c | 0.83 ± 0.01 b | 64.5 ± 2.1 b |
| P | 12 | 186.4 ± 7.6 a | 202.9 ± 22.2 b | 0.79 ± 0.01 c | 101.4 ± 9.4 a |

| Group | n | TNF-α (µg/mL) | IL-1β (µg/mL) | IL-6 (µg/mL) | IL-10 (µg/mL) |
|---|---|---|---|---|---|
| N | 12 | 16.68 ± 0.20 bc | 18.70 ± 0.77 c | 17.64 ± 0.33 d | 20.40 ± 0.33 d |
| S | 12 | 20.65 ± 0.43 a | 20.78 ± 0.78 b | 23.68 ± 1.26 a | 22.38 ± 0.48 c |
| L | 12 | 15.72 ± 1.25 d | 18.55 ± 0.78 c | 21.20 ± 0.91 c | 22.48 ± 0.18 c |
| M | 12 | 17.53 ± 0.36 b | 21.46 ± 0.34 ab | 22.71 ± 1.80 ab | 23.54 ± 0.25 a |
| H | 12 | 14.37 ± 0.93 e | 16.38 ± 1.76 d | 21.93 ± 0.37 bc | 23.42 ± 0.32 ab |
| P | 12 | 16.57 ± 0.45 cd | 22.09 ± 0.13 a | 18.71 ± 0.56 d | 23.05 ± 0.31 b |
| Group | n | NO (µM) | AST(GOT) (unit/L) | ALT(GPT) (unit/L) | C-RP (ng/mL) |
|---|---|---|---|---|---|
| N | 12 | 23.8 ± 1.6 c | 27.1 ± 2.4 c | 31.3 ± 2.1 a | 167.0 ± 18.1 b |
| S | 12 | 39.3 ± 5.1 a | 48.9 ± 3.8 a | 46.6 ± 2.5 b | 227.0 ± 20.0 a |
| L | 12 | 33.5 ± 2.7 b | 43.1 ± 2.3 b | 40.2 ± 3.0 c | 211.5 ± 10.4 a |
| M | 12 | 25.7 ± 1.6 b | 40.5 ± 2.6 b | 36.3 ± 2.1 d | 220.9 ± 19.9 a |
| H | 12 | 24.7 ± 1.5 c | 25.0 ± 2.3 c | 27.4 ± 2.3 a | 181.4 ± 16.7 b |
| P | 12 | 25.0 ± 3.5 c | 16.6 ± 3.1 d | 19.6 ± 2.4 e | 188.5 ± 17.9 b |
3. Experimental Section
3.1. Preparation of Test Samples
3.2. Animal Treatment and Grouping
- (1)
- N: Normal group (i.p. normal saline), fed the chow diet.
- (2)
- S: Sepsis group (i.p. LPS, 15 mg/kg BW), fed the chow diet.
- (3)
- L: Sepsis group with low-dose (1%) wild bitter gourd lyophilized powder added, fed feed including 1% wild bitter gourd lyophilized powder.
- (4)
- M: Sepsis group with moderate-dose (2%) wild bitter gourd lyophilized powder added, fed feed including 2% wild bitter gourd lyophilized powder.
- (5)
- H: Sepsis group with high-dose (10%) wild bitter gourd lyophilized powder added, fed feed including 10% wild bitter gourd lyophilized powder.
- (6)
- P: Positive control group (i.p. PDTC, 50 mg/kg BW), fed the chow diet.
3.3. Blood Preparation and Biochemical Assay
3.4. Measurement of Splenocyte Cytokine Levels by ELISA
3.5. Western Blot Analysis
3.6. Statistical Analysis
4. Conclusions
Abbreviation
| WBG | Wild bitter gourd |
| LPS | Lipopolysaccharides |
| COX-2 | Cyclooxygenase-2 |
| TNF-α | Tumor necrosis factor-α |
| PGE2 | Prostaglandin E2 |
| PPARs | Peroxisome proliferator-activated receptors |
| NO | Nitric oxide |
| PDTC | Pyrrolidinedithiocarbamic acid ammonium salt |
| NEFA | Non-esterified fatty acid |
| GOT | Glutamate oxaloacetate transaminase |
| C-RP | C-reactive protein |
| GPT | Glutamate pyruvate transaminase |
| PVDF | Polyvinylidene fluoride |
| ECL | Enhanced chemiluminescence |
| NF-κB | Nuclear factor-kappaB |
| iNOS | Inducible nitric oxide synthase |
| MOF | Multiple organ failure |
| CLA | Conjugated linoleic acid |
| STAT | Signal transducers and activators of transcription |
| LPC | Lysophosphatidylcholine |
| AP-1 | Activator protein-1 |
| MMPs | Metalloproteinase |
Acknowledgments
Author Contributions
Appendix

Conflicts of Interest
References
- Chao, C.Y.; Yin, M.C.; Huang, C.J. Wild bitter gourd extract up-regulates mRNA expression of PPARα, PPARγ and their target genes in C57BL/6J mice. J. Ethnopharmacol. 2011, 135, 156–161. [Google Scholar] [CrossRef]
- Lii, C.K.; Chen, H.W.; Yun, W.T.; Liu, K.L. Suppressive effects of wild bitter gourd (Momordica charantia Linn. var. abbreviate ser.) fruit extracts on inflammatory responses in RAW264.7 macrophages. J. Ethnopharmacol. 2009, 122, 227–233. [Google Scholar] [CrossRef]
- Uebanso, T.; Arai, H.; Taketani, Y.; Fukaya, M.; Yamamoto, H.; Mizuno, A.; Uryu, K.; Hada, T.; Takeda, E. Extracts of Momordica charantia suppress postprandial hyperglycemia in rats. J. Nutr. Sci. Vitaminol. 2007, 53, 482–488. [Google Scholar] [CrossRef]
- Welihinda, J.; Karunanayake, E.H.; Sheriff, M.H. Effect of Momordica charantia on the glucose tolerance in maturity onset diabetes. J.Ethnopharmacol. 1986, 17, 277–282. [Google Scholar] [CrossRef]
- Krawinkel, M.B.; Keding, G.B. Bitter gourd (Momordica charantia): A dietary approach to hyperglycemia. Nutr. Rev. 2006, 74, 331–337. [Google Scholar] [CrossRef]
- Kobori, M.; Nakayama, H.; Fukushima, K.; Ohnishi-Kameyama, M.; Ono, H.; Fukushima, T.; Akimoto, Y.; Masumoto, S.; Yukizaki, C.; Hoshi, Y.; et al. Bitter gourd suppresses lipopolysaccharide-induced inflammatory responses. J. Agric. Food Chem. 2008, 56, 4004–4011. [Google Scholar] [CrossRef]
- Chuang, C.Y.; Hsu, C.; Chao, C.Y.; Wein, Y.S.; Kuo, Y.H.; Huang, C.J. Fractionation and identification of 9c, 11t, 13t-conjugated linolenic acid as an activator of PPARalpha in bitter gourd (Momordica charantia L.). J. Biomed. Sci. 2006, 13, 763–772. [Google Scholar] [CrossRef]
- Chang, C.I.; Tseng, H.I.; Liao, Y.W.; Yen, C.H.; Chen, T.M.; Lin, C.C.; Cheng, H.L. In vivo and in vitro studies to identify the hypoglycaemic constituents of Momordica charantia wild variant WB24. Food Chem. 2011, 125, 521–528. [Google Scholar] [CrossRef]
- Hsu, C.; Hsieh, C.L.; Kuo, Y.H.; Huang, C.J. Isolation and identification of cucurbitane-type triterpenoids with partial agonist/antagonist potential for estrogen receptors from Momordica charantia. J. Agric. Food Chem. 2011, 59, 4553–4561. [Google Scholar] [CrossRef]
- Hsu, C.; Tsai, T.H.; Li, Y.Y.; Wu, W.H.; Huang, C.J.; Tsai, P.J. Wild bitter melon (Momordica charantia Linn. var. abbreviata Ser.) extract and its bioactive components suppress Propionibacterium acnes-induced inflammation. Food Chem. 2012, 135, 976–984. [Google Scholar] [CrossRef]
- Leung, L.; Birtwhistle, R.; Kotecha, J.; Hannah, S.; Cuthbertson, S. Anti-diabetic and hypoglycaemic effects of Momordica charantia (bitter melon): A mini review. Brit. J. Nutr. 2009, 102, 1703–1708. [Google Scholar] [CrossRef]
- Weinberg, J.B. Nitric oxide synthase and cyclooxygenase 2 interactions in inflammation. Immunol. Res. 2000, 22, 319–341. [Google Scholar] [CrossRef]
- Nathan, C.F. Secretory products of macrophages. J. Clin. Investig. 1987, 79, 319–323. [Google Scholar] [CrossRef]
- Chao, C.Y.; Huang, C.J. Bitter gourd (momordica charantia) extract activates peroxisome proliferator-activated receptors and upregulates the expression of the acyl CoA oxidase gene in H4IIEC3 hepatoma cells. J. Biomed. Sci. 2003, 10, 782–791. [Google Scholar]
- Chinetti, G.; Fruchart, J.C.; Staels, B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm. Res. 2000, 49, 497–505. [Google Scholar] [CrossRef]
- Chung, J.H.; Seo, A.Y.; Chung, S.W.; Kim, M.K.; Leeuwenburgh, C.; Yu, B.P.; Chung, H.Y. Molecular mechanism of PPAR in the regulation of age-related inflammation. Ageing Res. Rev. 2008, 7, 126–136. [Google Scholar] [CrossRef]
- Reddy, R.C. Immunomodulatory role of PPAR-gamma in alveolar macrophages. J. Investig. Med. 2008, 56, 522–527. [Google Scholar]
- Maitra, S.R.; Wojnar, M.M.; Lang, C.H. Alterations in tissue glucose uptake during the hyperglycemic and hypoglycemic phases of sepsis. Shock 2000, 13, 379–385. [Google Scholar] [CrossRef]
- Huang, C.J.; Wu, M.C. Differential effects of foods traditionally regarded as ‘heating’ and ‘cooling’ on prostaglandin E2 production by a macrophage cell line. J. Biomed. Sci. 2002, 9, 596–606. [Google Scholar]
- Lee, S.S.; Pineau, T.; Drago, J.; Lee, E.J.; Owens, J.W.; Kroetz, D.L.; Fernandez-Salguero, P.M.; Westphal, H.; Gonzalez, F.J. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol. Cell. Biol. 1995, 15, 3012–3022. [Google Scholar]
- Yumiko, Y.; Masashi, H.; Takehiko, S.; Rikako, S.; Satoru, O.; Hiroyuki, K.; Takuji, T.; Kazuo, M. Bitter gourd seed fatty acid rich in 9c,11t,13t-conjugated linolenic acid induces apoptosis and up-regulates the GADD45, p53 and PPARγ in human colon cancer Caco-2 cells. Prostaglandins Leukot. Essent. Fatty Acids 2005, 73, 113–119. [Google Scholar] [CrossRef]
- Rodriguez, J.C.; Gil-Gomez, G.; Hegardt, F.G.; Haro, D. Peroxisome proliferator-activated receptor mediates induction of the mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene by fatty acids. J. Biol. Chem. 1994, 269, 18767–18772. [Google Scholar]
- Mukherjee, R.; Jow, L.; Noonan, D.; McDonnell, D.P. Human and rat peroxisome proliferator activated receptors (PPARs) demonstrate similar tissue distribution but different responsiveness to PPAR activators. J. Steroid Biochem. 1994, 51, 157–166. [Google Scholar] [CrossRef]
- Yukihiro, S. Liver in systemic disease. World J. Gastroenterol. 2008, 14, 4111–4119. [Google Scholar] [CrossRef]
- Surh, Y.J.; Chun, K.S.; Cha, H.H.; Han, S.S.; Keum, Y.S.; Park, K.K.; Lee, S.S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat. Res. 2001, 480, 243–268. [Google Scholar]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Gardiner, S.M.; Kemp, P.A.; March, J.E.; Bennett, T. Cardiac and regional haemodynamics, inducible nitric oxide synthase (iNOS) activity, and the effects of NOS inhibitors in conscious, endotoxaemic rats. Brit. J. Pharmacol. 1995, 116, 2005–2016. [Google Scholar] [CrossRef]
- Hanada, T.; Yoshimura, A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 2002, 13, 413–421. [Google Scholar] [CrossRef]
- Ricote, M.; Li, A.C.; Willson, T.M.; Kelly, C.J.; Glass, C.K. Peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 1998, 391, 79–82. [Google Scholar] [CrossRef]
- Iwasashi, H.; Suzuki, M.; Unno, M.; Utiyama, T.; Oikawa, M.; Kondo, N.; Matsuno, S. Inhibition of heme oxygenase ameliorates sepsis-induced liver dysfunction in rats. Surg. Today 2003, 3, 30–38. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chao, C.-Y.; Sung, P.-J.; Wang, W.-H.; Kuo, Y.-H. Anti-Inflammatory Effect of Momordica Charantia in Sepsis Mice. Molecules 2014, 19, 12777-12788. https://doi.org/10.3390/molecules190812777
Chao C-Y, Sung P-J, Wang W-H, Kuo Y-H. Anti-Inflammatory Effect of Momordica Charantia in Sepsis Mice. Molecules. 2014; 19(8):12777-12788. https://doi.org/10.3390/molecules190812777
Chicago/Turabian StyleChao, Che-Yi, Ping-Jyun Sung, Wei-Hsien Wang, and Yueh-Hsiung Kuo. 2014. "Anti-Inflammatory Effect of Momordica Charantia in Sepsis Mice" Molecules 19, no. 8: 12777-12788. https://doi.org/10.3390/molecules190812777
APA StyleChao, C.-Y., Sung, P.-J., Wang, W.-H., & Kuo, Y.-H. (2014). Anti-Inflammatory Effect of Momordica Charantia in Sepsis Mice. Molecules, 19(8), 12777-12788. https://doi.org/10.3390/molecules190812777