Effects of Endogenous Signals and Fusarium oxysporum on the Mechanism Regulating Genistein Synthesis and Accumulation in Yellow Lupine and Their Impact on Plant Cell Cytoskeleton
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Effect of Sucrose, Glucose and Fructose on Accumulation of Genistein and Other Isoflavones in Embryo Axes Infected with F. oxysporum
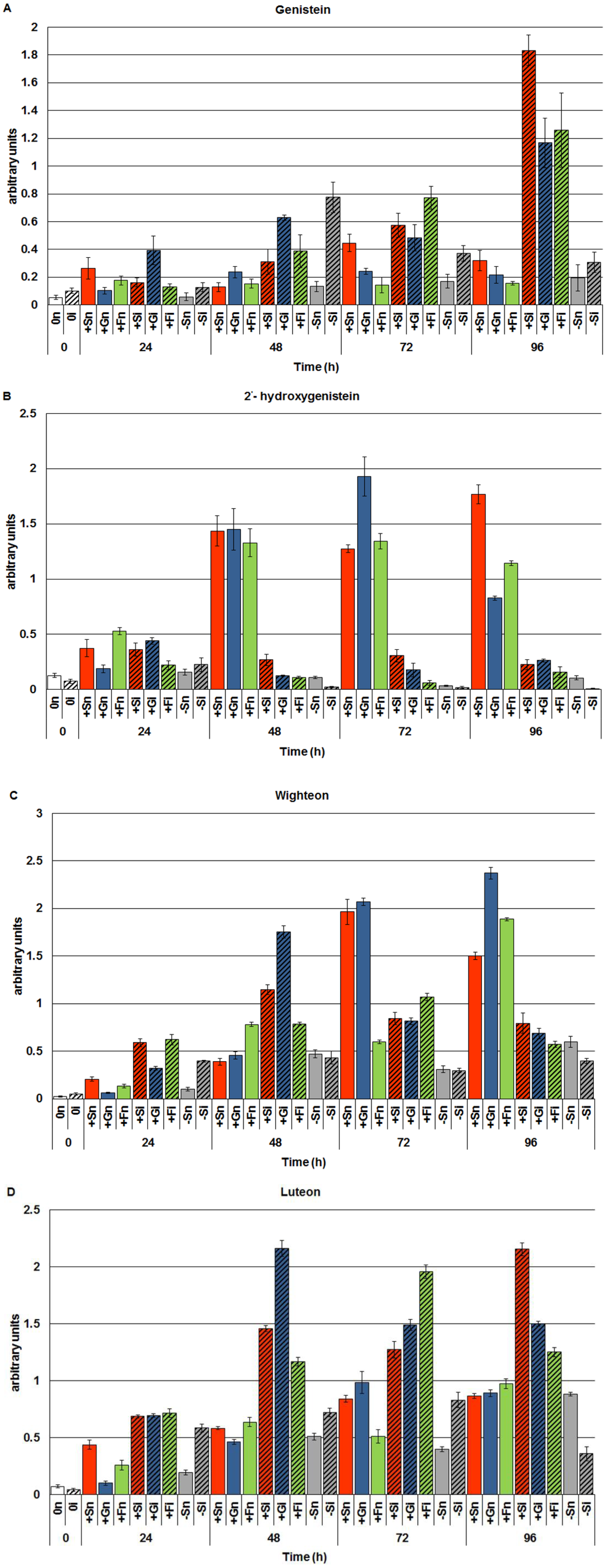
2.2. The Effect of Sucrose, Glucose and Fructose on Expression Levels of Genistein Biosynthesis Pathway Genes in Response to Infection with F. oxysporum

2.3. The Effect of Exogenous Sucrose, Glucose and Fructose on Endogenous Levels of Soluble Sugars and Their Changes in Response to Infection with F. oxysporum

2.4. The Effect of Sucrose, Glucose and Fructose on β-Glucosidase Activity in Response to Infection with F. oxysporum

2.5. The Effect of Sucrose, Glucose and Fructose on Superoxide Anion Radical (O2•−) Generation in Response to Infection with F. oxysporum

2.6. The Effect of Sucrose, Glucose and Fructose on Semiquinone Radical Generation in Response to Infection with F. oxysporum
2.7. The Effect of Sucrose on Actin and Tubulin Cytoskeleton Organization in Response to Infection with F. oxysporum



2.8. Discussion
3. Experimental Section
3.1. Materials
3.1.1. Plant Material and Growth Conditions
3.1.2. Preparation of Spore Suspension and Inoculation
3.2. Methods
3.2.1. Analysis of Isoflavonoids
3.2.1.1. Isolation of Phenolic Compounds
3.2.1.2. Liquid Chromatography (LC/UV/MS)
3.2.2. Real-time RT-PCR
| Protein Name | Primer Name | Sequence (5'-3') | Amplicon (bp) |
|---|---|---|---|
| chalcone synthase | CHS F | ATCCTGATTTCTACTTCAGA | 160 |
| CHS R | GGTGCCATATAAGCACAAA | ||
| phenylalanine ammonia-lyase | PAL F | ATTTAACTCTGTACCATTGCCG | 132 |
| PAL R | GGAGAACCAAACAGGGCG | ||
| chalcone isomerase | CHI F | AGAATCAGCTGAGAAATGATA | 207 |
| CHI R | GAGAAGGTTGTTAGACTTGT | ||
| isoflavone synthase | IFS F | TGGGTTGTTGATGAGCTCTG | 162 |
| IFS R | GTTTTTCTTGATACTTTGCTTG | ||
| actin | Actin F | TGGTCGTCCTCGTCACACT | 72 |
| Actin R | TGTGCCTCATCCCCAACATA |
3.2.3. Extraction and Assay of β-glucosidase Activity
3.2.4. Electron Paramagnetic Resonance (EPR)
3.2.5. Determination of Superoxide Anion Radical Content
3.2.6. Carbohydrate Analysis
Extraction
Derivatisation
GC-MS Analyses
3.2.7. Detection of Actin and Tubulin Cytoskeleton
3.2.8. Statistical Analysis
4. Conclusions
Abbreviations
| +Sn | embryo axes non-inoculated and cultured in vitro on Heller’s medium supplemented with 60 mM sucrose |
| +Gn | embryo axes non-inoculated and cultured in vitro on Heller’s medium supplemented with 120 mM glucose |
| +Fn | embryo axes non-inoculated and cultured in vitro on Heller’s medium supplemented with 120 mM fructose |
| −Sn | non-inoculated cultured without sucrose |
| +Si | inoculated and cultured with 60 mM sucrose |
| +Gi | inoculated and cultured with 120 mM glucose |
| +Fi | inoculated and cultured with 120 mM fructose |
| −Si | inoculated and cultured without sucrose |
| CHI | chalcone isomerase |
| CHS | chalcone synthase |
| EPR | electron paramagnetic resonance |
| IFS | isoflavone synthase |
| PAL | phenylalanine ammonia-lyase |
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dixon, R.A.; Ferreira, D. Molecules of interest: Genistein. Phytochemistry 2002, 60, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Rusin, A.; Krawczyk, Z.; Grynkiewicz, G.; Gogler, A.; Zawisza-Puchalka, J.; Szeja, W. Synthetic derivatives of genistein, their properties and possible applications. Acta Biochim. Pol. 2010, 57, 23–34. [Google Scholar] [PubMed]
- Maćkowiak, P.; Nogowski, L.; Nowak, K.W. Effect of isoflavone genistein on insulin receptors in perfused liver of ovariectomized rats. J. Recept. Signal. Transduct. Res. 1999, 19, 283–292. [Google Scholar]
- Szkudelski, T.; Nogowski, L.; Pruszyńska-Oszmałek, E.; Kaczmarek, P.; Szkudelska, K. Genistein restricts leptin secretion from rat adipocytes. J. Steroid Biochem. Mol. Biol. 2005, 96, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.M.; Markham, K.R. Flavonoids: Chemistry, Biochemistry, and Applications; CRC Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Graham, T.L.; Graham, M.Y. Defense potentiation and elicitation competency: Redox conditioning effects of salicylic acid and genistein. In Plant Microbe Interactions; Stacey, G., Keen, N.T., Eds.; APS Press: St. Paul, MN, USA, 2000; Volume 5, pp. 181–220. [Google Scholar]
- Groβkinsky, D.K.; van der Graaff, E.; Roitsch, T. Phytoalexin transgenics in crop protection-Fairy tale with a happy end? Trends Plant Sci. 2012, 195, 54–70. [Google Scholar]
- Ribera, A.E.; Zuñiga, G. Induced plant secondary metabolites for phytopatogenic fungi control: A review. J. Soil Sci. Plant Nutr. 2012, 12, 893–911. [Google Scholar]
- Morkunas, I.; Marczak, Ł.; Stachowiak, J.; Stobiecki, M. Sucrose-stimulated accumulation of isoflavonoids as a defense response of lupine to Fusarium oxysporum. Plant Physiol. Biochem. 2005, 43, 363–373. [Google Scholar]
- Kosslak, R.M.; Rookland, R.; Barkei, J.; Paaren, H.E.; Appelbaum, E.R. Induction of Bradyrhizobium japonicum common nod genes by isoflavones isolated from Glycine max. Proc. Natl. Acad. Sci. USA 1987, 84, 7428–7432. [Google Scholar] [CrossRef] [PubMed]
- Dolatabadian, A.; Sanavy, S.A.M.M.; Ghanati, F.; Gresshoff, P.M. Morphological and physiological response of soybean treated with the microsymbiont Bradyrhizobium japonicum pre-incubated with genistein. S. Afr. J. Bot. 2012, 79, 9–18. [Google Scholar] [CrossRef]
- Smith, D.A.; Banks, S. Biosynthesis, elicitation and biological activity of isoflavonoid phytoalexins (review). Phytochemistry 1986, 25, 979–995. [Google Scholar] [CrossRef]
- Kneer, R.; Poulev, A.A.; Olesinski, A.; Raskin, I. Characterization of the elicitor-induced biosynthesis and secretion of genistein from roots of Lupinus luteus L. J. Exp. Bot. 1999, 50, 1553–1559. [Google Scholar] [CrossRef]
- Morkunas, I.; Stobiecki, M.; Marczak, Ł.; Stachowiak, J.; Narożna, D.; Remlein-Starosta, D. Changes in carbohydrate and isoflavonoid metabolism in yellow lupine in response to infection by Fusarium oxysporum during the stages of seed germination and early seedling growth. Physiol. Mol. Plant Pathol. 2010, 75, 46–55. [Google Scholar]
- Bednarek, P.; Kerhoas, L.; Einhorn, J.; Frański, R.; Wojtaszek, P.; Rybus-Zajac, M.; Stobiecki, M. Profiling of flavonoid conjugates in Lupinus albus and Lupinus angustifolius responding to biotic and abiotic stimuli. J. Chem. Ecol. 2003, 29, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Gębicki, J.; Grynkiewicz, G. Radical scavenging properties of genistein. Free Radic. Biol. Med. 2003, 35, 958–965. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.M.; Guy, G.W.; di Marzo, V. Care and feeding of the endocannabinoid system: A systematic review of potential clinical interventions that upregulate the endocannabinoid system. PLoS One 2014, 9, e89566. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Flavonoids: Advances in Research since 1986; Harborne, J.B., Ed.; Chapman & Hall: London, UK, 1994; p. 152. [Google Scholar]
- Hoffmann, D. Medical herbalism: The Science and Practice of Herbal Medicine; Healing Arts Press: Rochester, VT, USA, 2003; p. 106. [Google Scholar]
- Deavours, B.E.; Dixon, R.A. Metabolic engineering of isoflavonoid biosynthesis in alfalfa. Plant Physiol. 2005, 138, 2245–2259. [Google Scholar] [CrossRef] [PubMed]
- Kessmann, H.; Choudhary, A.D.; Dixon, R.A. Stress responses in alfalfa (Medicago sativa L.) III. Induction of medicarpin and cytochrome P450 enzyme activities in elicitor-treated cell suspension cultures and protoplasts. Plant Cell Rep. 1990, 9, 38–41. [Google Scholar]
- Dixon, R.A. Phytoestrogens. Annu. Rev. Plant Biol. 2004, 55, 225–261. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Clément, C.; Courot, E.; Cordelier, S. Modulation of phytoalexin biosynthesis in engineered plants for disease resistance. Int. J. Mol. Sci. 2013, 14, 14136–14170. [Google Scholar] [CrossRef] [PubMed]
- Shinde, A.N.; Malpathak, N.; Fulzele, D.P. Impact of nutrient components on production of the phytoestrogens daidzein and genistein by hairy roots of Psoralea corylifolia. J. Nat. Med. 2010, 64, 346–353. [Google Scholar]
- Baque, M.A.; Elgirban, A.; Lee, E.J.; Paek, K.Y. Sucrose regulated enhanced induction of anthraquinone, phenolics, flavonoids biosynthesis and activities of antioxidant enzymes in adventitious root suspension cultures of Morinda citrifolia (L.). Acta Physiol. Plant. 2012, 34, 405–415. [Google Scholar]
- Hara, M.; Oki, K.; Hoshino, K.; Kuboi, T. Enhancement of anthocyanin biosynthesis by sugar in radish (Raphanus sativus) hypocotyls. Plant Sci. 2003, 164, 259–265. [Google Scholar] [CrossRef]
- Solfanelli, C.; Poggi, A.; Loreti, E.; Alpi, A.; Perata, P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 2006, 140, 637–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morkunas, I.; Narożna, D.; Nowak, W.; Samardakiewicz, S.; Remlein-Starosta, D. Cross-talk interactions of sucrose and Fusarium oxysporum in the phenylpropanoid pathway and the accumulation and localization of flavonoids in embryo axes of yellow lupine. J. Plant Physiol. 2011, 168, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; van den Ende, W.; Rolland, F. Sucrose induction of anthocyanin biosynthesis is mediated by DELLA. Mol. Plant 2014, 7, 570–572. [Google Scholar] [CrossRef] [PubMed]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [PubMed]
- Bolouri-Moghaddam, M.R.; Le Roy, K.; Xiang, L.; Rolland, F.; van den Ende, W. Sugar signalling and antioxidant network connections in plant cells. FEBS J. 2010, 277, 2022–2037. [Google Scholar]
- Bolouri Moghaddam, M.R.; van den Ende, W. Sugars and plant innate immunity. J. Exp. Bot. 2012, 63, 3989–3998. [Google Scholar]
- Shinde, A.N.; Malpathak, N.; Fulzele, D.P. Studied enhancement strategies for phytoestrogens production in shake flasks by suspension culture of Psoralea corylifolia. Bioresour. Technol. 2009, 100, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.B.; Li, H.Q.; Wan, X.C.; Jiang, C.J. Effect of several physiochemical factors on cell growth and isoflavone accumulation of Pueraria lobata cell suspension culture. China J. Chin. Mater. Med. 2006, 31, 1580–1583. [Google Scholar]
- Pasqua, G.; Monacelli, B.; Mulinacci, N.; Rinaldi, S.; Giaccherini, C.; Innocenti, M.; Vinceri, F.F. The effect of growth regulators and sucrose on anthocyanin production in Camptotheca acuminatacell cultures. Plant Physiol. Biochem. 2005, 43, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.; Keurentjes, J.; Bentsink, L.; Koornneef, M.; Smeekens, S. Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol. 2005, 139, 1840–1852. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Formela, M.; Floryszak-Wieczorek, J.; Marczak, Ł.; Narożna, D.; Nowak, W.; Bednarski, W. Cross-talk interactions of exogenous nitric oxide and sucrose modulates phenylpropanoid metabolism in yellow lupine embryo axes infected with Fusarium oxysporum. Plant Sci. 2013, 211, 102–121. [Google Scholar]
- Wojakowska, A.; Muth, D.; Narożna, D.; Mądrzak, C.; Stobiecki, M.; Kachlicki, P. Changes of phenolic secondary metabolite profiles in the reaction of narrow leaf lupin (Lupinus angustifolius) plants to infections with Colletotrichum lupini fungus or treatment with its toxin. Metabolomics. 2013, 9, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Muth, D.; Kachlicki, P.; Krajewski, P.; Przystalski, M.; Stobiecki, M. Differential metabolic response of narrow leafed lupine (Lupinus angustifolius) leaves to infection with Colletotrichum lupini. Metabolomics 2009, 5, 354–362. [Google Scholar] [CrossRef]
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Grayer, R.J.; Kokubun, T. Plant-fungal interactions: The search for phytoalexins and other antifungal compounds from higher plants. Phytochemistry 2001, 56, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Kozłowska, M.; Ratajczak, L.; Marczak, Ł. Role of sucrose in the development of Fusarium wilt in lupine embryo axes. Physiol. Mol. Plant Pathol. 2007, 70, 25–37. [Google Scholar] [CrossRef]
- Higaki, T.; Kutsuna, N.; Sano, T.; Kondo, N.; Hasezawa, S. Quantification and cluster analysis of actin cytoskeletal structures in plant cells: Role of actin bundling in stomatal movement during diurnal cycles in Arabidopsis guard cells. Plant J. 2010, 61, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Hardham, A.R. Microtubules and biotic interactions. Plant J. 2013, 75, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Nick, P. Microtubules and the tax payer. Protoplasma 2012, 249, 81–94. [Google Scholar] [CrossRef]
- Samardakiewicz, S.; Krzesłowska, M.; Woźny, A. Tubulin cytoskeleton in plant cell response to trace metals. In Compartmentation of Responses to Stresses in Higher Plants, True or False; Maksymiec, R., Ed.; Transword Research Network: Trivandrum, India, 2009; pp. 149–162. [Google Scholar]
- Staiger, C.J.; Sheahan, M.B.; Khurana, P.; Wang, X.; McCurdy, D.W.; Blanchoin, L. Actin filament dynamics are dominated by rapid growth and severing activity in the Arabidopsis cortical array. J. Cell Biol. 2009, 184, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.Y.; Yao, L.L.; Jia, Z.Q.; Li, Y.; Li, Y.Z. Verticillium dahliae toxin induced alterations of cytoskeletons and nucleoli in Arabidopsis thaliana suspension cells. Protoplasma 2006, 229, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Henty-Ridilla, J.L.; Shimono, M.; Li, J.; Chang, J.H.; Day, B.; Staiger, C.J. The plant actin cytoskeleton responds to signals from microbe-associated molecular patterns. PLoS Pathog. 2013, 9, e1003290. [Google Scholar]
- Wojtaszek, P. Cytoszkielet. In Biologia Komórki Roślinnej. Struktura; Wojtaszek, P., Woźny, A., Ratajczak, L., Eds.; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2006; Volume 1, pp. 194–226. [Google Scholar]
- Nick, P. Mechanics of the cytoskeleton. In Mechanical Integration of Plant Cells and Plants; Wojaszek, P., Ed.; Springer: Berlin, Germany, 2011; pp. 53–90. [Google Scholar]
- Hardham, A.R.; Jones, D.A.; Takemoto, D. Cytoskeleton and cell wall function in penetration resistance. Curr. Opin. Plant Biol. 2007, 10, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Delorme-Axford, E.; Coyne, C.B. The actin cytoskeleton as a barrier to virus infection of polarized epithelial cells. Viruses 2011, 3, 2462–2477. [Google Scholar]
- Navarro-Garcia, F.; Serapio-Palacios, A.; Ugalde-Silva, P.; Tapia-Pastrana, G.; Chavez-Dueñas, L. Actin cytoskeleton manipulation by effector proteins secreted by diarrheagenic Escherichia coli pathotypes. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Smertenko, A.; Franklin-Tong, V.E. Organisation and regulation of the cytoskeleton in plant programmed cell death. Cell. Death Differ. 2011, 18, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Zadworny, M.; Guzicka, M.; Łakomy, P.; Samardakiewicz, S.; Smoliński, D.J.; Mucha, J. Analysis of microtubule and microfilament distribution in Pinus sylvestris roots following infection by Heterobasidion species. For. Pathol. 2013, 43, 222–231. [Google Scholar]
- Wojtaszek, P.; Baluška, F.; Kasprowicz, A.; Łuczak, M.; Volkmann, D. Domain-specific mechanosensory transmission of osmotic and enzymatic cell wall disturbances to the actin cytoskeleton. Protoplasma 2007, 230, 217–230. [Google Scholar]
- Banaś, A.K.; Krzeszowiec, W.; Dobrucki, J.; Gabryś, H. Mannose, but not glucose or sucrose, disturbs actin cytoskeleton in Arabidopsis thaliana leaves. Acta Physiol. Plant. 2010, 32, 773–779. [Google Scholar]
- Balasubramanian, R.; Karve, A.; Kandasamy, M.; Meagher, R.B.; Moore, B. A role for F-actin in hexokinase-mediated glucose signaling. Plant Physiol. 2007, 145, 1423–1434. [Google Scholar]
- Winter, H.; Huber, J.L.; Huber, S.C. Identification of sucrose synthase as an actin-binding protein. FEBS Lett. 1998, 430, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Kasprowicz, A.; Szuba, A.; Volkmann, D.; Baluška, F.; Wojtaszek, P. Nitric oxide modulates dynamic actin cytoskeleton and vesicle trafficking in a cell type-specific manner in root apices. J. Exp. Bot. 2009, 60, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- Heller, R. Recherches sur la nutrition minerale des tissues vegetaux cultives in vitro. Ann. Sci. Nat. Bot. Biol. Veg. 1954, 14, 1–223. [Google Scholar]
- Morkunas, I.; Gmerek, J. The possible involvement of peroxidase in defense of yellow lupine embryo axes against Fusarium oxysporum. J. Plant Physiol. 2007, 164, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Bednarski, W. Fusarium oxysporum induced oxidative stress and antioxidative defenses of yellow lupine embryo axes with different level of sugars. J. Plant Physiol. 2008, 165, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Frański, R.; Bednarek, P.; Wojtaszek, P.; Stobiecki, M. Identification of flavonoid diglycosides in yellow lupin (Lupinus luteus L.) with mass spectrometric techniques. J. Mass Spectrom. 1999, 344, 486–495. [Google Scholar]
- Nichols, E.J.; Beckman, J.M.; Hadwiger, L.A. Glycosidic enzyme activity in pea tissue and pea-Fusarium solani interaction. Plant Physiol. 1980, 66, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Garnczarska, M.; Bednarski, W.; Ratajczak, W.; Waplak, S. Metabolic and ultrastructural responses of lupine embryo axes to sugar starvation. J. Plant Physiol. 2003, 160, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Bednarski, W.; Kozłowska, M. Response of embryo axes of germinating seeds of yellow lupine to Fusarium oxysporum. Plant Physiol. Biochem. 2004, 42, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Bednarski, W.; Kopyra, M. Defense strategies of pea embryo axes with different levels of sucrose to Fusarium oxysporum and Ascochyta pisi. Physiol. Mol. Plant Pathol. 2008, 72, 167–178. [Google Scholar] [CrossRef]
- Bednarski, W.; Ostrowski, A.; Waplak, S. Low temperature short-range ordering caused by Mn2+doping of Rb3H(SO4)2. J. Phys. Condens. Matter 2010, 22, 225901. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.C.; Bednarski, W.; Borowiak-Sobkowiak, B.; Wilkaniec, B.; Samardakiewicz, S.; Morkunas, I. Oxidative stress in pea seedling leaves in response to Acyrthosiphon pisum infestation. Phytochemistry 2013, 93, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Doke, N. Generation of superoxide anion by potato tuber protoplasts during hypersensitive response to hyphal wall components of Phytophtora infestans and specific inhibition of the reaction with supressors of hypersensitivity. Physiol. Plant Pathol. 1983, 23, 359–367. [Google Scholar] [CrossRef]
- Olyslaegers, G.; Verbelen, J.P. Improved staining of F-actin and co-localization of mitochondria in plant cells. J. Microsc. 1998, 192, 73–77. [Google Scholar] [CrossRef]
- Vitha, S.; Baluška, F.; Mews, M.; Volkmann, D. Immunofluorescence detection of F-actin on low melting point wax sections from plant tissues. J. Histochem. Cytochem. 1997, 45, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Formela, M.; Samardakiewicz, S.; Marczak, Ł.; Nowak, W.; Narożna, D.; Bednarski, W.; Kasprowicz-Maluśki, A.; Morkunas, I. Effects of Endogenous Signals and Fusarium oxysporum on the Mechanism Regulating Genistein Synthesis and Accumulation in Yellow Lupine and Their Impact on Plant Cell Cytoskeleton. Molecules 2014, 19, 13392-13421. https://doi.org/10.3390/molecules190913392
Formela M, Samardakiewicz S, Marczak Ł, Nowak W, Narożna D, Bednarski W, Kasprowicz-Maluśki A, Morkunas I. Effects of Endogenous Signals and Fusarium oxysporum on the Mechanism Regulating Genistein Synthesis and Accumulation in Yellow Lupine and Their Impact on Plant Cell Cytoskeleton. Molecules. 2014; 19(9):13392-13421. https://doi.org/10.3390/molecules190913392
Chicago/Turabian StyleFormela, Magda, Sławomir Samardakiewicz, Łukasz Marczak, Witold Nowak, Dorota Narożna, Waldemar Bednarski, Anna Kasprowicz-Maluśki, and Iwona Morkunas. 2014. "Effects of Endogenous Signals and Fusarium oxysporum on the Mechanism Regulating Genistein Synthesis and Accumulation in Yellow Lupine and Their Impact on Plant Cell Cytoskeleton" Molecules 19, no. 9: 13392-13421. https://doi.org/10.3390/molecules190913392






