Evaluation of the Antioxidant, Anti-Inflammatory, and Anticancer Activities of Euphorbia hirta Ethanolic Extract
Abstract
:1. Introduction
2. Results and Discussion
2.1. Gas Chromatography-Mass Spectrometry (GC-MS) of Ethanolic Extract of E. hirta

| S. No. | RT a | Name of Compounds b | Molecular Formula | MW | Area (%) c |
|---|---|---|---|---|---|
| 1 | 9.66 | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one | C6H8O4 | 144 | 2.54 |
| 2 | 12.06 | 5-Hydroxymethyl-2-furancarboxaldehyde | C6H6O3 | 126 | 7.82 |
| 3 | 16.39 | 1,2,3-Trihydroxybenzene | C6H6O3 | 126 | 3.72 |
| 4 | 25.51 | Myristic acid | C14H28O2 | 228 | 1.03 |
| 5 | 30.48 | Pentadecylic acid | C15H30O2 | 242 | 13.27 |
| 6 | 31.34 | Ethyl palmitate | C18H36O2 | 284 | 7.47 |
| 7 | 35.11 | Phytol | C20H40O | 296 | 3.93 |
| 8 | 35.81 | Methyl linoleate | C19H34O2 | 294 | 7.29 |
| 9 | 36.00 | 9,12,15-Octadecatrien-1-ol | C18H32O | 264 | 18.56 |
| 10 | 36.47 | 7,10-Octadecadienoic acid methyl ester | C19H34O2 | 294 | 6.22 |
| 11 | 36.65 | Ethyl linoleolate | C20H34O2 | 306 | 7.7 |
| 12 | 37.25 | Ethyl stearate | C20H40O2 | 312 | 2.18 |
| 13 | 48.78 | Ethyl hexadecanoate | C18H36O2 | 284 | 0.44 |
| 14 | 49.43 | Squalene | C30H50 | 410 | 1.63 |
| 15 | 54.41 | gamma-Tocopherol | C28H48O2 | 417 | 0.76 |
| 84.56 # |
2.2. Antioxidant Activity


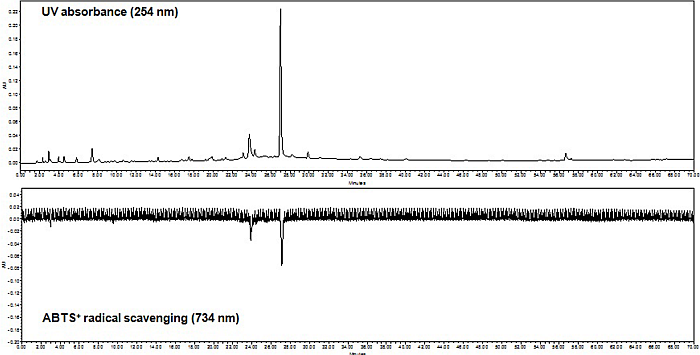
2.3. On-Line HPLC-ABTS+• Radical Scavenging Analysis

2.4. Effects of E. hirta on Lipopolysaccharide (LPS)-Induced NO Production

2.5. Cytotoxicity Assessment of E. hirta Extract on Cell Lines in Vitro

2.6. Anticancer Activity against an Acute Myeloid Leukemia Cell Line (HL-60) in Vitro

3. Experimental Section
3.1. Plant Specimen and Preparation of Ethanolic Extract
3.2. GC-MS Analysis
3.3. DPPH Radical Scavenging Assay
3.4. OH˙ Scavenging Assay
3.5. On-Line HPLC-ABTS+• Radical Scavenging Analysis
3.6. Cell Culture
3.7. Determination of NO Production
3.8. Cytotoxicity Assessment by MTT Assay
3.9. Anticancer Activity Assay
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gruenwald, J. The Global Herbs & Botanicals Market. Available online: http://www.nutraceuticalsworld.com/issues/2008-07/view_features/the-global-herbs-amp-botanicals-market/ (accessed on 10 December 2013).
- Kumar, A.S.; Reddy, T.S.K. Importance of traditional system of medicine—A review. Int. J. Phytother. 2012, 2, 1–6. [Google Scholar]
- Bansal, A.K.; Bilaspuri, G.S. Impacts of oxidative stress and antioxidants on semen functions. Vet. Med. Int. 2010, 2010. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Salles, B.; Sattler, U.; Bozzato, C.; Calsou, P. Repair of oxidative DNA damage in vitro: A tool for screening antioxidative compounds. Food Chem. Toxicol. 1999, 37, 1009–1014. [Google Scholar] [CrossRef]
- Jae-Ha, R.; Hanna, A.; Hwa-Jin, L.; Wen-He, Q.; Yong-Nam, H.; Byung-Hoon, H. Inhibitory activity of Chinese medicinal plants on nitric oxide synthesis in lipopolysaccharide activated macrophages. J. Appl. Pharmacol. 2001, 9, 183–187. [Google Scholar]
- Shih, M.F.; Cheng, Y.D.; Shen, C.R.; Cherng, J.Y. A molecular pharmacology study into the anti-inflammatory actions of Euphorbia hirta L. on the LPS-induced RAW 264.7 cells through selective iNOS protein inhibition. J. Nat. Med 2011, 64, 330–335. [Google Scholar]
- Lee, J.K. Anti-inflammatory effects of eriodictyol in lipopolysaccharide-stimulated raw 264.7 murine macrophages. Arch. Pharmacal. Res. 2011, 34, 671–679. [Google Scholar] [CrossRef]
- Ban, J.O.; Hwang, I.G.; Kim, T.M.; Hwang, B.Y.; Lee, U.S.; Jeong, H.S.; Yoon, Y.W.; Kim, D.J.; Hong, J.T. Anti-proliferate and pro-apoptotic effects of 2,3-Dihydro-3,5- dihydroxy-6-methyl-4H-pyranone through inactivation of NF-κB in human colon cancer cells. Arch. Pharm. Res. 2007, 30, 1455–1463. [Google Scholar]
- Brustugun, J.; Tonnesen, H.H.; Edge, R.; Navaratnam, S. Formation and reactivity of free radicals in 5-hydroxymethyl-2-furaldehyde–the effect on isoprenaline photostability. J. Photochem. Photobiol. B 2005, 79, 109–119. [Google Scholar] [CrossRef]
- Li, Y.H.; Lu, X.Y. Investigation on the origin of 5-HMF in Shengmaiyin decoction by RP-HPLC method. J. Zhejiang Univ. Sci. B 2005, 6, 1015–1021. [Google Scholar] [CrossRef]
- Xu, Q.; Li, Y.H.; Lu, X.Y. Investigation on influencing factors of 5-HMF content in Schisandra. J. Zhejiang Univ. Sci. B 2007, 8, 439–345. [Google Scholar]
- Graff, C.L.; Pollack, G.M. Nasal drug administration: Potential for targeted central nervous system delivery. J. Pharma. Sci. 2005, 94, 1187–1195. [Google Scholar] [CrossRef]
- Kagoura, M.; Matsui, C.; Morohashi, M. Phytol is a novel tumor promoter on ICR mouse skin. Jpn. J. Cancer Res. 1999, 90, 377–384. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Z.; Wang, S.; Wang, S.; Liu, J. Antioxidant and antibacterial activities of Camptotheca acuminate D.seed oil. Afr. J. Microbiol. Res. 2011, 5, 5854–5862. [Google Scholar]
- Nordberg, J.; Arner, E.S.J. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Heo, S.J.; Cha, S.H.; Lee, K.W.; Jeon, Y.J. Antioxidant activities of red algae from Jeju Island. Algae 2006, 21, 149–156. [Google Scholar] [CrossRef]
- Kitada, M.; Igaradhi, K.; Hirose, S.; Kitagawa, H. Inhibition bypolyamines of lipid peroxidase formation in rat liver microsomes. Biochem. Biophys. Res. Commun. 1979, 87, 388–394. [Google Scholar] [CrossRef]
- Osawa, T. Novel natural antioxidants for utilization in food and biological systems. In Postharvest Biochemistry of Plant Food-materials in the Tropics; Uritani, I., Garcia, V.V., Mendoza, E.M., Eds.; Japan Scientific Societies Press: Tokyo, Japan, 1994; pp. 241–251. [Google Scholar]
- Kant, K.; Walia, M.; Agnihotri, V.K.; Pathania, V.; Singh, B. Evaluation of antioxidant activity Picrorhiza kurroa (leaves) extracts. Indian J. Pharm. Sci. 2013, 75, 324–329. [Google Scholar] [CrossRef]
- Basma, A.A.; Zakaria, Z.; Latha, L.Y.; Sasidharan, S. Antioxidant activity and phytochemical screening of the methanol extracts of Euphorbia hirta L. Asian Pac. J. Trop. Med. 2011, 386–390. [Google Scholar]
- Senthilkumar, S.; Devaki, T.; Manohar, B.M.; Babu, M.S. Effect of squalene on cyclophosphamide-induced toxicity. Clin. Chim. Acta 2006, 364, 335–342. [Google Scholar] [CrossRef]
- Dorfman, L.M.; Adams, G.E. Reactivity of the hydroxyl radical in aqueous solutions. National Bureau of Standards. 1973, pp. 1–59. Available online: http://www.nist.gov/data/nsrds/NSRDS-NBS-46.pdf (accessed on 15 December 2013).
- Rosen, G.M.; Rauckman, E.J. Spin trapping of superoxide and hydroxyl radicals. Methods Enzymol. 1984, 105, 198–209. [Google Scholar]
- Liu, C.H.; Huang, H.Y. Antimicrobial activity of curcumin-loaded myristic acid microemulsions against Staphylococcus epidermidis. Chem. Pharm. Bull. 2012, 60, 1118–1124. [Google Scholar] [CrossRef]
- Van der Veen, R.C. Nitric oxide and T helper cell immunity. Int. Immunopharmacol. 2001, 1, 1491–1500. [Google Scholar] [CrossRef]
- Lefkowitz, D.L.; Gelderman, M.P.; Fuhrmann, S.R.; Graham, S.; Starnes, J.D.; Lefkowitz, S.S.; Bollen, A.; Moguilevsky, N. Neutrophilic lysozyme-macrophage interactions perpetuate chronic inflammation associated with experimental arthritis. Clin. Immunol. 1999, 91, 145–155. [Google Scholar] [CrossRef]
- Hausladen, A.; Stamler, J.S. Nitrosative stress. Methods Enzymol. 1999, 300, 389–395. [Google Scholar] [CrossRef]
- Murphy, M.P. Nitric oxide and cell death. Biochim. Biophys. Acta 1411, 401–414. [Google Scholar]
- Clancy, R.M.; Amin, A.R.; Abramson, S.B. The role of nitric oxide in inflammation and immunity. Arthritis Rheumatol. 1998, 41, 1141–1151. [Google Scholar]
- Moncada, S.; Palmer, R.M.J.; Higgs, E.A. Nitric oxide physiology, pathophysiology and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar]
- Goyal, R.; Sharma, P.L.; Singh, M. Pharmacological potential of Tecomella undulate in acute and chronic inflammation in rat. Int. J. Pharm. Sci. Rev. Res. 2010, 1, 108–114. [Google Scholar]
- Xu, B.D.; Chen, K.; Lin, W.J.; Zhang, H.L. Comparative study on chemical components of supercitical extracts from Herba ephedrae and honey-prepared Herba ephedrae. J. Guangzhou Univ. TCM 2004, 21, 211–212. [Google Scholar]
- Miyazawa, M.; Anzai, J.; Fujioka, J.; Isikawa, Y. Insecticidal compounds against Drosophila melanogaster from Cornus officinalis Sieb. Et Zucc. Nat. Prod. Res. 2003, 17, 337–339. [Google Scholar]
- Sharma, V.K.; Choi, J.; Sharma, N.; Choi, M.; Seo, S.Y. In vitro anti-tyrosinase activity of 5-(hydroxymethyl)-2-furfural isolated from Dictyophora indusiata. Phytother. Res. 2004, 18, 841–844. [Google Scholar]
- Hou, Y.C.; Ching, H.; Chao, P.D.L.; Tsai, S.Y.; Wen, K.C.; Hsieh, P.H.; Hsiu, S.L. Effects of glucose, fructose and 5-hydroxymethyl-2-furaldehyde on the presystemic metabolism and absorption of glycyrrhizin in rabbits. J. Pharm. Pharmacol. 2005, 57, 247–251. [Google Scholar] [CrossRef]
- Vijaya, K.; Ananthan, S.; Nalini, R. Antibacterial effect of theaflavin, polyphenon 60 (Camellia sinensis) and Euphorbia hirta on Shigella spp.—A cell culture study. J. Ethnopharmacol. 1995, 49, 115–118. [Google Scholar] [CrossRef]
- Sidambaram, R.R.; Dinesh, M.G.; Jayalakshmi, E.T. An in vitro study of cytotoxic activity of euphorbia hirta on hep2 cells of human epithelioma of larynx. Int. J. Pharm. Pharm. Sci. 2011, 3, 101–103. [Google Scholar]
- Aggarwal, S.; Ichikawa, H.; Takada, Y.; Sandur, S.K.; Shishodia, S.; Aggarwal, B.B. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of Ikappa Balpha kinase and Akt activation. Mol. Pharmacol. 2006, 69, 195–206. [Google Scholar]
- Liang, M.C.; Bardhan, S.; Pace, E.A.; Rosman, D.; Beutler, J.A.; Porco, J.A.; Gilmore, T.D. Inhibition of transcription factor NF-κB signaling proteins IKKbeta and p65 through specific cysteine residues by epoxyquinone A monomer: Correlation with its anticancer cell growth activity. Biochem. Pharmacol. 2006, 71, 634–645. [Google Scholar] [CrossRef]
- Shishodia, S.; Aggarwal, B.B. Nuclear factor-κB: A friend or a foe in cancer? Biochem. Pharmacol. 2004, 68, 1071–1080. [Google Scholar]
- Hardwick, J.C.H.; van den Brink, G.R.; Offerhaus, G.J.; van Deventer, S.J.; Peppelenbosch, M.P. NF-κB, p38 MAPK and JNK are highly expressed and active in the stroma of human colonic adenomatous polyps. Oncogene 2001, 20, 819–827. [Google Scholar]
- Nanjo, F.; Goto, K.; Seto, R.; Suzuki, M.; Sakai, M.; Hara, Y. Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylydrazyl radical. Free Radic. Biol. Med. 1996, 21, 895–902. [Google Scholar] [CrossRef]
- Hur, S.J.; Choi, S.Y.; Lim, B.O. In vitro anti-inflammatory activity of Russula virescens in the macrophage like cell line RAW 264.7 activated by lipopolysaccharide. J. Nutr. Food Sci. 2012, 2. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the whole plant powder of E. hirta are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sharma, N.; Samarakoon, K.W.; Gyawali, R.; Park, Y.-H.; Lee, S.-J.; Oh, S.J.; Lee, T.-H.; Jeong, D.K. Evaluation of the Antioxidant, Anti-Inflammatory, and Anticancer Activities of Euphorbia hirta Ethanolic Extract. Molecules 2014, 19, 14567-14581. https://doi.org/10.3390/molecules190914567
Sharma N, Samarakoon KW, Gyawali R, Park Y-H, Lee S-J, Oh SJ, Lee T-H, Jeong DK. Evaluation of the Antioxidant, Anti-Inflammatory, and Anticancer Activities of Euphorbia hirta Ethanolic Extract. Molecules. 2014; 19(9):14567-14581. https://doi.org/10.3390/molecules190914567
Chicago/Turabian StyleSharma, Neelesh, Kalpa W. Samarakoon, Rajendra Gyawali, Yang-Ho Park, Sung-Jin Lee, Sung Jong Oh, Tae-Hoon Lee, and Dong Kee Jeong. 2014. "Evaluation of the Antioxidant, Anti-Inflammatory, and Anticancer Activities of Euphorbia hirta Ethanolic Extract" Molecules 19, no. 9: 14567-14581. https://doi.org/10.3390/molecules190914567
APA StyleSharma, N., Samarakoon, K. W., Gyawali, R., Park, Y.-H., Lee, S.-J., Oh, S. J., Lee, T.-H., & Jeong, D. K. (2014). Evaluation of the Antioxidant, Anti-Inflammatory, and Anticancer Activities of Euphorbia hirta Ethanolic Extract. Molecules, 19(9), 14567-14581. https://doi.org/10.3390/molecules190914567