Anti-Inflammatory and Analgesic Effects of the Marine-Derived Compound Comaparvin Isolated from the Crinoid Comanthus bennetti
Abstract
:1. Introduction

2. Results
2.1. Cell Viability

2.2. Effects of Comaparvin on LPS-Induced iNOS Protein Expression
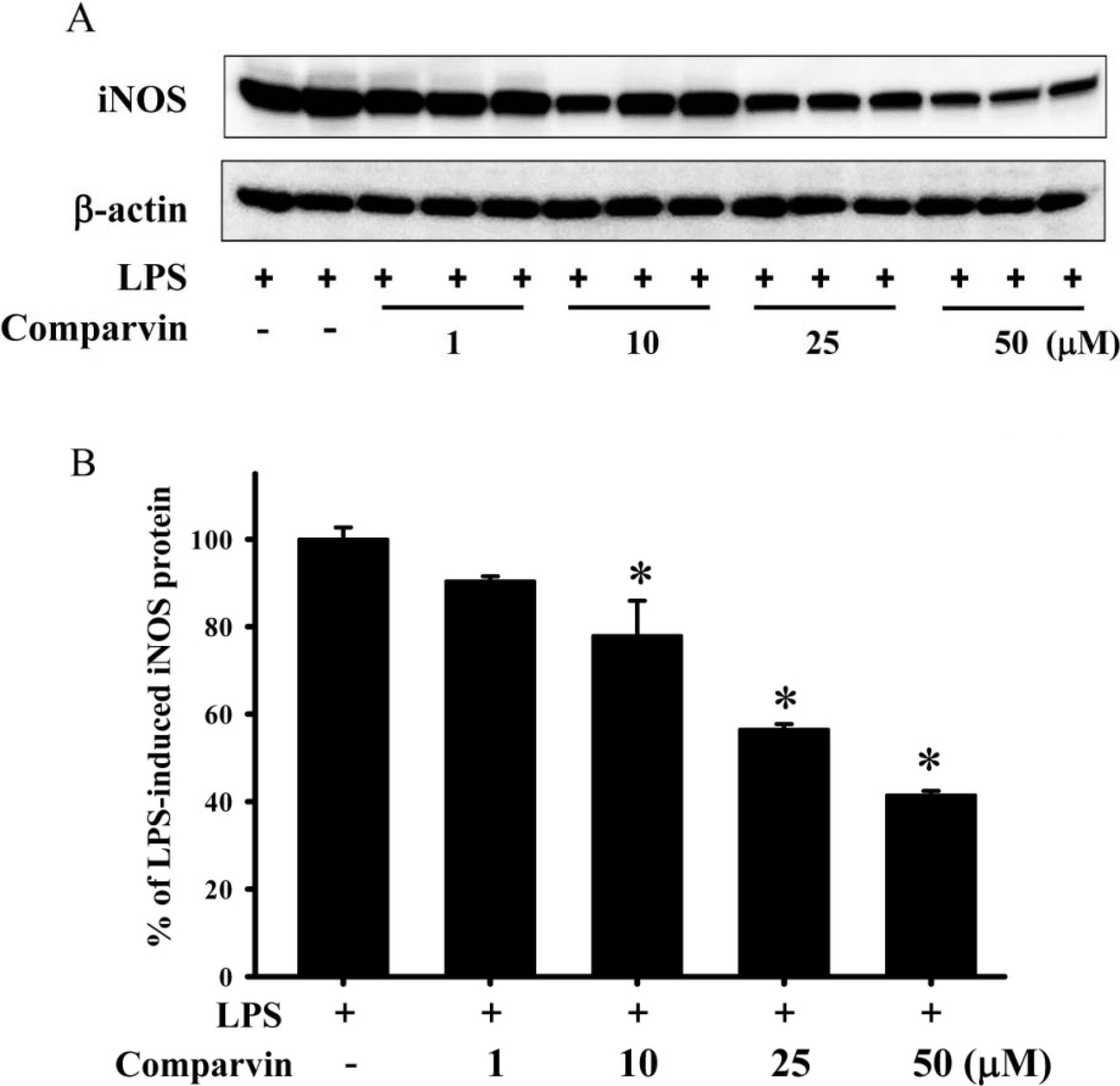
2.3. Effects of Comaparvin on LPS-Induced iNOS mRNA Expression

2.4. Effects of Comaparvin on Carrageenan-Induced Weight-Bearing Defects

2.5. Effects of Comaparvin on Carrageenan-Induced Thermal Hyperalgesia

2.6. Effects of Comaparvin on Carrageenan-Induced Mechanical Allodynia

2.7. Effect of Comaparvin on Carrageenan-Induced Cell Infiltration in Paw Tissue
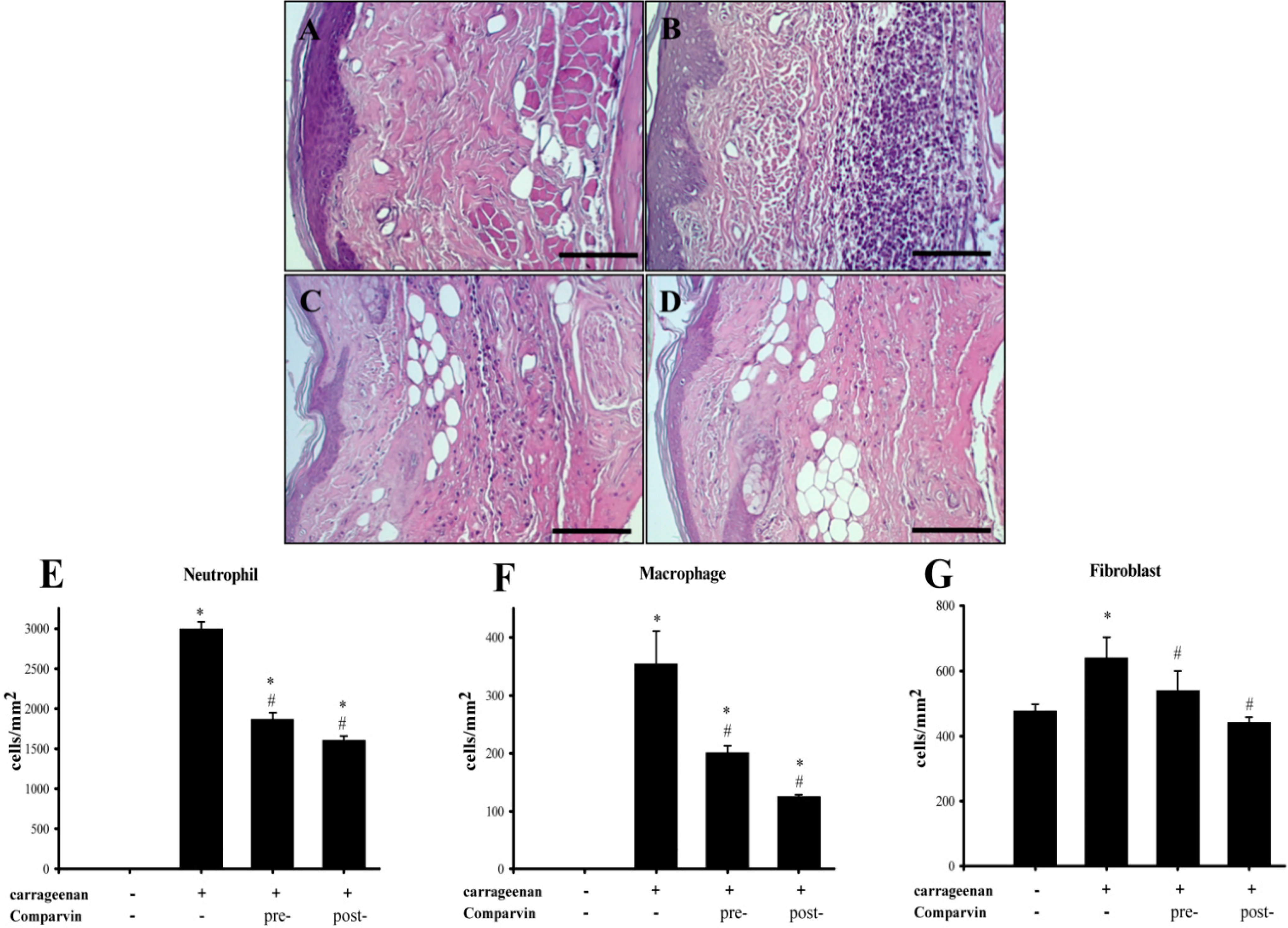
2.8. Effects of Comaparvin on Carrageenan-Induced iNOS Expression in Inflamed Paw Tissue

3. Discussion
4. Experimental Section
4.1. Extraction of Comaparvin
4.2. Anti-Inflammatory Assay in Vitro
4.2.1. Cell Culture
4.2.2. Western Blotting for iNOS
4.2.3. Quantitative Polymerase Chain Reaction (qPCR) Analyses for iNOS mRNA in LPS-Stimulated RAW Cells
4.3. Carrageenan-Induced Inflammation and Pain
4.3.1. Preparation of Animals
4.3.2. Design of Animal Experiments
4.3.3. Analyses of the Pain Behavior of Rats
Weight-Bearing
Plantar Thermal Hyperalgesia
Mechanical Allodynia
4.4. Preparation of Paw Tissues for Western Blot Analysis
4.5. Histopathologic Analyses
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mayer, A.M.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology in 2009–2011: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2013, 11, 2510–2573. [Google Scholar] [CrossRef] [Green Version]
- Ebada, S.S.; Edrada, R.A.; Lin, W.; Proksch, P. Methods for isolation, purification and structural elucidation of bioactive secondary metabolites from marine invertebrates. Nat. Protoc. 2008, 3, 1820–1831. [Google Scholar]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar]
- Senthilkumar, K.; Kim, S.K. Marine invertebrate natural products for anti-Inflammatory and chronic diseases. Evid. Based Complement. Altern. Med. 2013, 2013, 572859. [Google Scholar]
- Aneiros, A.; Garateix, A. Bioactive peptides from marine sources: Pharmacological properties and isolation procedures. J. Chromatogr. B. Anal. Technol. Biomed. Life Sci. 2004, 803, 41–53. [Google Scholar] [CrossRef]
- Bolker, H.I. Crinosterol: A unique sterol from a comatulid crinoids. Nature 1967, 213, 905–906. [Google Scholar]
- Smith, I.R.; Sutherland, M.D. Pigments of marine animals. Aust. J. Chem. 1971, 24, 1487–1499. [Google Scholar] [CrossRef]
- Bokesch, H.R.; Cartner, L.K.; Fuller, R.W.; Wilson, J.A.; Henrich, C.J.; Kelley, J.A.; Gustafson, K.R.; McMahon, J.B.; McKeea, T.C. Inhibition of ABCG2-mediated drug efflux by naphthopyrones from marine crinoids. Bioorg. Med. Chem. Lett. 2010, 20, 3848–3850. [Google Scholar] [CrossRef]
- Chovolou, Y.; Ebada, S.S.; Wätjen, W.; Proksch, P. Identification of angular naphthopyrones from the philippine echinoderm Comanthus species as inhibitors of the NF-κB signaling pathway. Eur. J. Pharmacol. 2011, 657, 26–34. [Google Scholar]
- Laille, M.; Gerald, F.; Debitus, C. In vitro antiviral activity on dengue virus of marine natural products. Cell. Mol. Life Sci. 1998, 54, 167–170. [Google Scholar]
- Dai, J.; Liu, Y.; Jia, H.; Zhou, Y.D.; Nagle, D.G. Benzochromenones from the marine crinoid comantheria rotula inhibit hypoxia-inducible factor-1 (HIF-1) in cell-based reporter assays and differentially suppress the growth of certain tumor cell lines. J. Nat. Prod. 2007, 70, 1462–1466. [Google Scholar] [CrossRef]
- Shao, N.; Yao, G.; Chang, L.C. Bioactive constituents from the marine crinoid Himerometra magnipinna. J. Nat. Prod. 2007, 70, 869–871. [Google Scholar]
- Folmer, F.; Harrison, W.T.; Tabudravu, J.N.; Jaspars, M.; Aalbersberg, W.; Feussner, K.; Wright, A.D.; Dicato, M.; Diederich, M. NF-kappaB-inhibiting naphthopyrones from the fijian echinoderm Comanthus parvicirrus. J. Nat. Prod. 2008, 71, 106–111. [Google Scholar]
- Karin, M.; Yamamoto, Y.; Wang, Q.M. The IKK NF-kappa B system: A treasure trove for drug development. Nat. Rev. Drug Discov. 2004, 3, 17–26. [Google Scholar]
- Ren, T.; Qiu, Y.; Wu, W.; Feng, X.; Ye, S.; Wang, Z.; Tian, T.; He, Y.; Yu, C.; Zhou, Y. Activation of adenosine A3 receptor alleviates TNF-α-induced inflammation through inhibition of the NF-κB signaling pathway in human colonic epithelial cells. Mediat. Inflamm. 2014, 2014, 818251. [Google Scholar]
- Wang, T.; Guo, M.; Song, X.; Zhang, Z.; Jiang, H.; Wang, W.; Fu, Y.; Cao, Y.; Zhu, L.; Zhang, N. Stevioside plays an anti-inflammatory role by regulating the NF-κB and MAPK pathways in S. aureus-infected mouse mammary glands. Inflammation 2014, 2014, 1–10. [Google Scholar]
- Beck, K.F.; Eberhardt, W.; Frank, S.; Huwiler, A.; Messmer, U.K.; Mühl, H.; Pfeilschifter, J. Inducible NO synthase: Role in cellular signalling. J. Exp. Biol. 1999, 202, 645–653. [Google Scholar]
- García-Mediavilla, V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar]
- Seibert, K.; Zhang, Y.; Leahy, K.; Hauser, S.; Masferrer, J.; Perkins, W.; Lee, L.; Isakson, P. Pharmacolgical and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc. Natl. Acad. Sci. USA 1994, 91, 12013–12017. [Google Scholar]
- Salvemini, D.; Wang, Z.Q.; Wyatt, P.S.; Bourdon, D.M.; Marino, M.H.; Manning, P.T.; Currie, M.G. Nitric oxide: A key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br. J. Pharmacol. 1996, 118, 829–838. [Google Scholar]
- Li, Q.; Verma, I.M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar]
- Cheng, H.; Wang, L.; Mollica, M.; Re, A.T.; Wu, S.; Zuo, L. Nitric oxide in cancer metastasis. Cancer Lett. 2014, 353, 1–7. [Google Scholar] [CrossRef]
- Huang, S.Y.; Chen, N.F.; Chen, W.F.; Hung, H.C.; Lee, H.P.; Lin, Y.Y.; Wang, H.M.; Sung, P.J.; Sheu, J.H.; Wen, Z.H. Sinularin from indigenous soft coral attenuates nociceptive responses and spinal neuroinflammation in carrageenan-induced inflammatory rat model. Mar. Drugs 2012, 10, 1899–1919. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar]
- Sekiguchi, F.; Mita, Y.; Kamanaka, Y.; Kawao, N.; Matsuya, H.; Taga, C.; Kawabata, A. The potent inducible nitric oxide synthase inhibitor ONO-1714 inhibits neuronal NOS and exerts antinociception in rats. Neurosci. Lett. 2004, 365, 111–115. [Google Scholar]
- Coruzzi, G.; Adami, M.; Guaita, E.; de Esch, I.J.; Leurs, R. Antiinflammatory and antinociceptive effects of the selective histamine H4-receptor antagonists JNJ7777120 and VUF6002 in a rat model of carrageenan-induced acute inflammation. Eur. J. Pharmacol. 2007, 563, 240–244. [Google Scholar] [CrossRef]
- Jean, Y.H.; Chen, W.F.; Duh, C.Y.; Huang, S.Y.; Hsu, C.H.; Lin, C.S.; Sung, C.S.; Chen, I.M.; Wen, Z.H. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory and analgesic effects of the natural marine compound lemnalol from Formosan soft coral Lemnalia cervicorni. Eur. J. Pharmacol. 2008, 578, 323–331. [Google Scholar]
- Radhika, P.; Rao, P.R.; Archana, J.; Rao, N.K. Anti-inflammatory activity of a new sphingosine derivative and cembrenoid diterpene (Lobohedleolide) isolated from marine soft corals of sinularia crassa TIXIER-DURIVAULT and lobophytum species of the andaman and nicobar islands. Biol. Pharm. Bull. 2005, 28, 1311–1313. [Google Scholar]
- Cuzzocrea, S.; Mazzon, E.; Paola, R.D.; Peli, A.; Bonato, A.; Britti, D.; Genovese, T.; Muià, C.; Crisafulli, C.; Caputi, A.P. The role of the peroxisome proliferator-activated receptor-α (PPAR-α) in the regulation of acute inflammation. J. Leukoc. Biol. 2006, 79, 999–1010. [Google Scholar]
- Yen, Y.T.; Tu, P.H.; Chen, C.J.; Lin, Y.W.; Hsieh, S.T.; Chen, C.C. Role of acid-sensing ion channel 3 in sub-acute-phase inflammation. Mol. Pain 2009, 5. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar]
- Ribeiro, R.V.; da Silva, R.M.; da Silva Lima, J.C.; de Oliveira Martins, D.T. Antiinflammatory, antinociceptive and antipyretic effects of hydroethanolic extract from Macrosiphonia velame (A. St.-Hil.) M. Arg. in animal models. Braz. J. Pharm. Sci. 2010, 46, 515–523. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2009, 26, 170–244. [Google Scholar]
- Ho, F.M.; Lai, C.C.; Huang, L.J.; Kuo, T.C.; Chao, C.M.; Lin, W.W. The anti-inflammatory carbazole, LCY-2-CHO, inhibits lipopolysaccharide-induced inflammatory mediator expression through inhibition of thep38 mitogen- activated protein kinase signaling pathway in macrophages. Br. J. Pharmacol. 2004, 141, 1037–1047. [Google Scholar]
- Park, J.S.; Woo, M.S.; Kim, S.Y.; Kim, W.K.; Kim, H.S. Repression of interferon-gamma-induced inducible nitric oxide synthase (iNOS) gene expression in microglia by sodium butyrate is mediated through specific inhibition of ERK signaling pathways. J. Neuroimmunol. 2005, 168, 56–64. [Google Scholar]
- Lin, Y.C.; Huang, S.Y.; Jean, Y.H.; Chen, W.F.; Sung, C.S.; Kao, E.S.; Wang, H.M.; Chakraborty, C.; Duh, C.Y.; Wen, Z.H. Intrathecal lemnalol, a natural marine compound obtained from formosan soft coral, attenuates nociceptive responses and the activity of spinal glial cells in neuropathic rats. Behav. Pharmacol. 2011, 22, 739–750. [Google Scholar]
- Altemeier, W.A.; Zhu, X.; Berrington, W.R.; Harlan, J.M.; Liles, W.C. Fas (CD95) induces macrophage proinflammatory chemokine production via a MyD88-dependent, caspase-independent pathway. J. Leukoc. Biol. 2007, 82, 721–728. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (−delta delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- De Gois, S.; Schafer, M.K.; Defamie, N.; Chen, C.; Ricci, A.; Weihe, E.; Varoqui, H.; Erickson, J.D. Homeostatic scaling of vesicular glutamate and GABA transporter expression in rat neocortical circuits. J. Neurosci. 2005, 25, 7121–7133. [Google Scholar]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar]
- Sample Availability: Available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, L.-C.; Lin, Y.-Y.; Jean, Y.-H.; Lu, Y.; Chen, W.-F.; Yang, S.-N.; Wang, H.-M.D.; Jang, I.-Y.; Chen, I.-M.; Su, J.-H.; et al. Anti-Inflammatory and Analgesic Effects of the Marine-Derived Compound Comaparvin Isolated from the Crinoid Comanthus bennetti. Molecules 2014, 19, 14667-14686. https://doi.org/10.3390/molecules190914667
Chen L-C, Lin Y-Y, Jean Y-H, Lu Y, Chen W-F, Yang S-N, Wang H-MD, Jang I-Y, Chen I-M, Su J-H, et al. Anti-Inflammatory and Analgesic Effects of the Marine-Derived Compound Comaparvin Isolated from the Crinoid Comanthus bennetti. Molecules. 2014; 19(9):14667-14686. https://doi.org/10.3390/molecules190914667
Chicago/Turabian StyleChen, Li-Chai, Yen-You Lin, Yen-Hsuan Jean, Yi Lu, Wu-Fu Chen, San-Nan Yang, Hui-Min David Wang, Ing-Yang Jang, I-Ming Chen, Jui-Hsin Su, and et al. 2014. "Anti-Inflammatory and Analgesic Effects of the Marine-Derived Compound Comaparvin Isolated from the Crinoid Comanthus bennetti" Molecules 19, no. 9: 14667-14686. https://doi.org/10.3390/molecules190914667




