Highly Stable Tetra-Phenolato Titanium(IV) Agent Formulated into Nanoparticles Demonstrates Anti-Tumoral Activity and Selectivity
Abstract
:1. Introduction

2. Results and Discussion
2.1. Cytotoxicity toward Cisplatin Resistant Cells

| Complex | IC50 (μM) | Resistance Factor a | |
|---|---|---|---|
| A2780 | A2780-cp | ||
| LTi | 9 ± 1 | 10 ± 4 | 1.1 |
| Cisplatin | 3 ± 2 | 33 ± 2 | 11 |
| Carboplatin | 21 ± 14 | 210 ± 90 | 10 |
2.2. In Vitro Combination with Platinum Drugs

2.3. Activity Following Incubation in Cell Growth Media

2.4. Selectivity to Cancer Cells

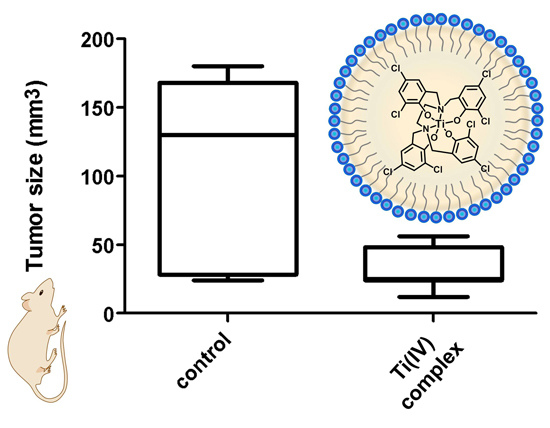
2.5. In Vivo Anti-Tumor Activity

3. Experimental Section
3.1. Ligand and Complex Synthesis
3.2. Materials
3.3. In Vitro Cytotoxicity Assays
3.4. Activity Following Incubation in Cell Growth Media
3.5. In Vivo Anti-Tumor Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ott, I.; Gust, R. Non platinum metal complexes as anti-cancer drugs. Arch. Pharm. 2007, 340, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Tshuva, E.Y.; Ashenhurst, J.A. Cytotoxic titanium(IV) complexes: Renaissance. Eur. J. Inorg. Chem. 2009, 2203–2218. [Google Scholar] [CrossRef]
- Koepf-Maier, P.; Koepf, H. Non-platinum group metal antitumor agents. history, current status, and perspectives. Chem. Rev. 1987, 87, 1137–1152. [Google Scholar] [CrossRef]
- Köpf-Maier, P.; Köpf, H. Transition and main-group metal cyclopentadienyl complexes: Preclinical studies on a series of antitumor agents of different structural type. Bioinorg. Chem. 1988, 70, 103–185. [Google Scholar]
- Melendez, E. Titanium complexes in cancer treatment. Crit. Rev. Oncol. Hematol. 2002, 42, 309–315. [Google Scholar] [CrossRef]
- Kostova, I. Titanium and vanadium complexes as anticancer agents. Anticancer Agents Med. Chem. 2009, 9, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Rossi, M. Antitumor titanium compounds. Mini-Rev. Med. Chem. 2004, 4, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Rossi, M.; Pettinari, C. Anticancer titanium agents. Expert Opin. Ther. Pat. 2001, 11, 969–979. [Google Scholar] [CrossRef]
- Buettner, K.M.; Valentine, A.M. Bioinorganic chemistry of titanium. Chem. Rev. 2012, 112, 1863–1881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Lippard, S.J. New metal complexes as potential therapeutics. Curr. Opin. Chem. Biol. 2003, 7, 481–489. [Google Scholar] [CrossRef]
- Bruijnincx, P.C.; Sadler, P.J. New trends for metal complexes with anticancer activity. Curr. Opin. Chem. Biol. 2008, 12, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Jakupec, M.A.; Galanski, M.; Arion, V.B.; Hartinger, C.G.; Keppler, B.K. Antitumour metal compounds: More than theme and variations. Dalton Trans. 2008, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Keppler, B.K.; Friesen, C.; Moritz, H.G.; Vongerichten, H.; Vogel, E. Tumor-inhibiting bis(beta-diketonato) metal-complexes—Budotitane, cis-diethoxybis(1-phenylbutane-1,3-dionato)titanium (IV)—The 1st transition-metal complex after platinum to qualify for clinical-trials. Struct. Bond. 1991, 78, 97–127. [Google Scholar]
- Desoize, B. Metals and metal compounds in cancer treatment. Anticancer Res. 2004, 24, 1529–1544. [Google Scholar] [PubMed]
- Muhammad, N.; Guo, Z. Metal-based anticancer chemotherapeutic agents. Curr. Opin. Chem. Biol. 2014, 19, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Arnesano, F.; Natile, G. Mechanistic insight into the cellular uptake and processing of cisplatin 30 years after its approval by FDA. Coord. Chem. Rev. 2009, 253, 2070–2081. [Google Scholar] [CrossRef]
- Muggia, F. Platinum compounds 30 years after the introduction of cisplatin: Implications for the treatment of ovarian cancer. Gynecol. Oncol. 2009, 112, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Kröger, N.; Kleeberg, U.R.; Mross, K.; Edler, L.; Hossfeld, D.K. Phase II clinical trial of titanocene dichloride in patients with metastatic breast cancer. Oncol. Res. Treat. 2000, 23, 60–62. [Google Scholar]
- Lümmen, G.; Sperling, H.; Luboldt, H.; Otto, T.; Rübben, H. Phase II trial of titanocene dichloride in advanced renal-cell carcinoma. Cancer Chemother. Pharmacol. 1998, 42, 415–417. [Google Scholar] [PubMed]
- Schilling, T.; Keppler, K.B.; Heim, M.E.; Niebch, G.; Dietzfelbinger, H.; Rastetter, J.; Hanauske, A.R. Clinical phase I and pharmacokinetic trial of the new titanium complex budotitane. Invest. New Drugs 1996, 13, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Massa, L.; Gindulyte, A.; Pettinari, C.; Marchetti, F.; Pettinari, R.; Ricciutelli, M.; Costamagna, J.; Canales, Juan C.; Tanski, J.; et al. (4-acyl-5-pyrazolonato)titanium derivatives: Oligomerization, hydrolysis, voltammetry, and dft study. Eur. J. Inorg. Chem. 2003, 2003, 3221–3232. [Google Scholar] [CrossRef]
- Toney, J.H.; Marks, T.J. Hydrolysis chemistry of the metallocene dichlorides M(.Eta.5-C5H5)2Cl2, M = titanium, vanadium, or zirconium. Aqueous kinetics, equilibria, and mechanistic implications for a new class of antitumor agents. J. Am. Chem. Soc. 1985, 107, 947–953. [Google Scholar] [CrossRef]
- Tshuva, E.Y.; Peri, D. Modern cytotoxic titanium(IV) complexes; insights on the enigmatic involvement of hydrolysis. Coord. Chem. Rev. 2009, 253, 2098–2115. [Google Scholar] [CrossRef]
- Peri, D.; Meker, S.; Shavit, M.; Tshuva, E.Y. Synthesis, characterization, cytotoxicity, and hydrolytic behavior of C-2- and C-1-symmetrical Ti-IV complexes of tetradentate diamine bis(phenolato) ligands: A new class of antitumor agents. Chem. Eur. J. 2009, 15, 2403–2415. [Google Scholar] [CrossRef] [PubMed]
- Shavit, M.; Peri, D.; Manna, C.M.; Alexander, J.S.; Tshuva, E.Y. Active cytotoxic reagents based on non-metallocene non-diketonato well-defined C-2-symmetrical titanium complexes of tetradentate bis(phenolato) ligands. J. Am. Chem. Soc. 2007, 129, 12098–12099. [Google Scholar] [CrossRef] [PubMed]
- Peri, D.; Meker, S.; Manna, C.M.; Tshuva, E.Y. Different ortho and para electronic effects on hydrolysis and cytotoxicity of diamino bis(phenolato) “Salan” Ti(IV) complexes. Inorg. Chem. 2011, 50, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Meker, S.; Manna, C.M.; Peri, D.; Tshuva, E.Y. Major impact of N-methylation on cytotoxicity and hydrolysis of salan Ti(IV) complexes: Sterics and electronics are intertwined. Dalton Trans. 2011, 40, 9802–9809. [Google Scholar] [CrossRef] [PubMed]
- Peri, D.; Manna, C.M.; Shavit, M.; Tshuva, E.Y. Ti-IV complexes of branched diamine bis(phenolato) ligands: Hydrolysis and cytotoxicity. Eur. J. Inorg. Chem. 2011, 2011, 4896–4900. [Google Scholar] [CrossRef]
- Tzubery, A.; Tshuva, E.Y. Trans titanium(IV) complexes of salen ligands exhibit high antitumor activity. Inorg. Chem. 2011, 50, 7946–7948. [Google Scholar] [CrossRef] [PubMed]
- Manna, C.M.; Braitbard, O.; Weiss, E.; Hochman, J.; Tshuva, E.Y. Cytotoxic salan-titanium(IV) complexes: High activity toward a range of sensitive and drug-resistant cell lines, and mechanistic insights. ChemMedChem 2012, 7, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Immel, T.A.; Grutzke, M.; Spate, A.K.; Groth, U.; Ohlschlager, P.; Huhn, T. Synthesis and x-ray structure analysis of a heptacoordinate titanium(IV)-bis-chelate with enhanced in vivo antitumor efficacy. Chem. Commun. 2012, 48, 5790–5792. [Google Scholar] [CrossRef] [PubMed]
- Glasner, H.; Tshuva, E.Y. A marked synergistic effect in antitumor activity of salan titanium(IV) complexes bearing two differently substituted aromatic rings. J. Am. Chem. Soc. 2011, 133, 16812–16814. [Google Scholar] [CrossRef] [PubMed]
- Manna, C.M.; Armony, G.; Tshuva, E.Y. New insights on the active species and mechanism of cytotoxicity of salan-Ti(IV) complexes: A stereochemical study. Inorg. Chem. 2011, 50, 10284–10291. [Google Scholar] [CrossRef] [PubMed]
- Immel, T.A.; Groth, U.; Huhn, T. Cytotoxic titanium salan complexes: Surprising interaction of salan and alkoxy ligands. Chem. Eur. J. 2010, 16, 2775–2789. [Google Scholar] [CrossRef] [PubMed]
- Tzubery, A.; Tshuva, E.Y. Cytotoxicity and hydrolysis of trans-Ti(IV) complexes of salen ligands: Structure-activity relationship studies. Inorg. Chem. 2012, 51, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Meker, S.; Margulis-Goshen, K.; Weiss, E.; Braitbard, O.; Hochman, J.; Magdassi, S.; Tshuva, E.Y. Anti-proliferative activity of nano-formulated phenolato titanium(IV) complexes against cancer cells. ChemMedChem 2014, 9, 1294–1298. [Google Scholar] [CrossRef] [PubMed]
- Meker, S.; Margulis-Goshen, K.; Weiss, E.; Magdassi, S.; Tshuva, E.Y. High antitumor activity of highly resistant salan-titanium(IV) complexes in nanoparticles: An identified active species. Angew. Chem. Int. Ed. 2012, 51, 10515–10517. [Google Scholar] [CrossRef] [PubMed]
- Severin, G.W.; Nielsen, C.H.; Jensen, A.I.; Fonslet, J.; Kjær, A.; Zhuravlev, F. Bringing radiotracing to titanium-based antineoplastics: Solid phase radiosynthesis, pet and ex vivo evaluation of antitumor agent [45Ti](salan)Ti(dipic). J. Med. Chem. 2015, 58, 7591–7595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glasner, H.; Meker, S.; Tshuva, E.Y. Cationic phenolato titanium(IV) complexes of enhanced solubility as active and biologically accessible anti-tumor compounds. J. Organomet. Chem. 2015, 788, 33–35. [Google Scholar] [CrossRef]
- Miller, M.; Tshuva, E.Y. Cytotoxic titanium(IV) complexes of chiral diaminobis(phenolato) ligands: Better combination of activity and stability by the bipyrrolidine moiety. Eur. J. Inorg. Chem. 2014, 2014, 1485–1491. [Google Scholar] [CrossRef]
- Margulis-Goshen, K.; Magdassi, S. Formation of simvastatin nanoparticles from microemulsion. Nanomedicine 2009, 5, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Margulis-Goshen, K.; Magdassi, S. Organic nanoparticles from microemulsions: Formation and applications. Curr. Opin. Colloid Interface Sci. 2012, 17, 290–296. [Google Scholar] [CrossRef]
- Higham, C.S.; Dowling, D.P.; Shaw, J.L.; Cetin, A.; Ziegler, C.J.; Farrell, J.R. Multidentate aminophenol ligands prepared with mannich condensations. Tetrahedron Lett. 2006, 47, 4419–4423. [Google Scholar] [CrossRef]
- Behrens, B.C.; Hamilton, T.C.; Masuda, H.; Grotzinger, K.R.; Whangpeng, J.; Louie, K.G.; Knutsen, T.; McKoy, W.M.; Young, R.C.; Ozols, R.F. Characterization of a cis-diamminedichloroplatinum(II)-resistant human ovarian-cancer cell-line and its use in evaluation of platinum analogs. Cancer Res. 1987, 47, 414–418. [Google Scholar] [PubMed]
- Masuda, H.; Ozols, R.F.; Lai, G.M.; Fojo, A.; Rothenberg, M.; Hamilton, T.C. Increased DNA-repair as a mechanism of acquired-resistance to cis-diamminedichloroplatinum(II) in human ovarian-cancer cell-lines. Cancer Res. 1988, 48, 5713–5716. [Google Scholar] [PubMed]
- Aird, R.E.; Cummings, J.; Ritchie, A.A.; Muir, M.; Morris, R.E.; Chen, H.; Sadler, P.J.; Jodrell, D.I. In vitro and in vivo activity and cross resistance profiles of novel ruthenium(II) organometallic arene complexes in human ovarian cancer. Brit. J. Cancer 2002, 86, 1652–1657. [Google Scholar] [CrossRef] [PubMed]
- Ganot, N.; Meker, S.; Reytman, L.; Tzubery, A.; Tshuva, E.Y. Anticancer metal complexes: Synthesis and cytotoxicity evaluation by the mtt assay. J. Vis. Exp. 2013, 81. [Google Scholar] [CrossRef] [PubMed]
- Rabik, C.A.; Dolan, M.E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 2007, 33, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Tallarida, R.J.; Porreca, F.; Cowan, A. Statistical-analysis of drug-drug and site-site interactions with isobolograms. Life Sci. 1989, 45, 947–961. [Google Scholar] [CrossRef]
- Ganot, N.; Redko, B.; Gellerman, G.; Tshuva, E.Y. Anti-proliferative activity of the combination of salan Ti(IV) complexes with other organic and inorganic anticancer drugs against HT-29 and NCI-H1229 cells: Synergism with cisplatin. Rsc. Adv. 2015, 5, 7874–7879. [Google Scholar] [CrossRef]
- Flick, D.A.; Gifford, G.E. Comparison of invitro-cell cyto-toxic assays for tumor necrosis factor. J. Immunol. Methods 1984, 68, 167–175. [Google Scholar] [CrossRef]
- Zhang, X.D.; Nguyen, T.; Thomas, W.D.; Sanders, J.E.; Hersey, P. Mechanisms of resistance of normal cells to trail induced apoptosis vary between different cell types. FEBS Lett. 2000, 482, 193–199. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meker, S.; Braitbard, O.; Margulis-Goshen, K.; Magdassi, S.; Hochman, J.; Tshuva, E.Y. Highly Stable Tetra-Phenolato Titanium(IV) Agent Formulated into Nanoparticles Demonstrates Anti-Tumoral Activity and Selectivity. Molecules 2015, 20, 18526-18538. https://doi.org/10.3390/molecules201018526
Meker S, Braitbard O, Margulis-Goshen K, Magdassi S, Hochman J, Tshuva EY. Highly Stable Tetra-Phenolato Titanium(IV) Agent Formulated into Nanoparticles Demonstrates Anti-Tumoral Activity and Selectivity. Molecules. 2015; 20(10):18526-18538. https://doi.org/10.3390/molecules201018526
Chicago/Turabian StyleMeker, Sigalit, Ori Braitbard, Katrin Margulis-Goshen, Shlomo Magdassi, Jacob Hochman, and Edit Y. Tshuva. 2015. "Highly Stable Tetra-Phenolato Titanium(IV) Agent Formulated into Nanoparticles Demonstrates Anti-Tumoral Activity and Selectivity" Molecules 20, no. 10: 18526-18538. https://doi.org/10.3390/molecules201018526