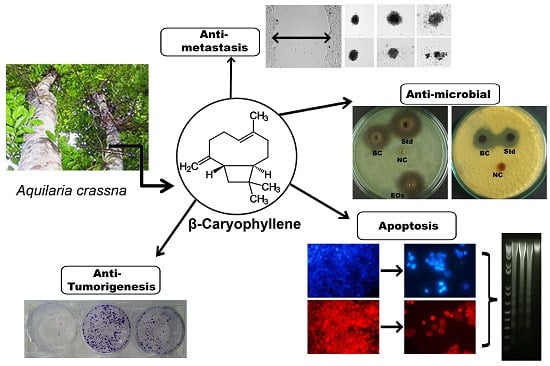
2.1. Extraction of Essential Oils and Isolation of the Active Principle
A. crassna is a plant with diverse traditional medicinal properties. A number of studies have confirmed that the essential oil is an active component of
A. crassna stem bark [
18,
19], but very little is known about the active principle(s) responsible for the pharmacological properties of the plant. The present study clearly demonstrated that the essential oil extracted from
A. crassna using the hydrodistillation method showed remarkable antiproliferative properties against human colorectal cancer cells. The main advantage of the hydrodistillation method over steam distillation is that less steam is used hence a shorter processing time is required and therefore the method gives a high yield of oil. This method is also less harsh on the botanical material and therefore the biological efficacy of the phytochemicals will be retained. Using repeated column chromatography on the essential oil, its fractions and subfractions were obtained, whereas the bioassay-guided screening resulted in the identification of the active principle of the essential oils (
Figure 1). The chemical structure of the active principle was elucidated using spectroscopic data that confirmed it was β-caryophyllene in nearly pure form (see
Supporting Information).
Figure 1.
Schematic diagram showing the bioassay (anti-proliferative assay)-guided isolation of β-caryophyllene from the essential oils of Aquilaria crassna.
Figure 1.
Schematic diagram showing the bioassay (anti-proliferative assay)-guided isolation of β-caryophyllene from the essential oils of Aquilaria crassna.
In the present study, the powdered material (500 g) of A. crassna stem bark was subjected to hydrodistillation to obtain the essential oil in a yield of 2.52%. The essential oil mixture showed significant anti-proliferation activity against HCT 116 (IC50 28 µg·mL−1), PANC-1 (IC50 32 µg·mL−1), PC3 (IC50 79 µg·mL−1) and MCF-7 (IC50 110 µg·mL−1) human cancer cell lines. The mixture of essential oils was subjected to column chromatography to obtain 12 fractions (F1-F12). Among all the fractions, fraction 8 (F8) showed most potent activity (HCT 116 IC50 11 µg·mL−1; PANC-1 IC50 18 µg·mL−1; PC3 IC50 26 µg·mL−1 and MCF-7 IC50 72 µg·mL−1). Further chromatographic separation of the fraction 8 yielded three sub-fractions (SF1-SF3). Among the three sub-fractions, SF3 was found to be the most active one against the proliferation of HCT 116 (IC50 6.3 µg·mL−1) and PANC-1 (IC50 11.7 µg·mL−1). Recrystallization of sub-fraction SF3 using hot methanol yielded β-caryophyllene.
2.2. Characterization of A. crassna Essential Oil and β-Caryophyllene Using GC-MS
A. crassna essential oil and the isolated compound β-caryophyllene were subjected to GC-MS analysis to quantify the major chemical constituents and confirm their molecular weights, respectively.
Figure 2 depicts the comparative GC-MS (
Figure 2A) of the extract and β-caryophyllene (
Figure 2B). The pie chart in
Figure 2A represents the composition and proportion of the major phytoconstituents present in
A. crassna essential oil. The GC-MS data such as retention time (R
t), % area peak, molecular formula and molecular weight obtained for the major chemical components are given in the
Supporting Information (
Table S1). In addition, the mass spectra for all the major chromatographic peaks identified are given in the
Supporting Information (
Figure S1A–M) are given in
Table S1. The chemical composition of the essential oil was identified using the NIST library. Accordingly, the GC-MS analysis revealed that the essential oil mixture was composed of various polyphenols and aromatic compounds. The quantitative analysis of the essential oil showed that the major peaks corresponded to β-caryophyllene (8.1%), 1-phenanthrenecarboxylic acid (7.1%), 2-naphthalene-methanol (6.2%), α-caryophyllene (4.7%), benzenedicarboxylic acid (4.6%), azulene (3.9%), naphthalene (2.7%) and cyclodecene (2.6%).
Figure 2.
Gas chromatograpic analysis of
Aquilaria crassna and β-caryophyllene. (
A) Chemical characterization of the essential oil of
Aquilaria crassna by GC-MS. The pie charts depict the relative chemical composition of the sub-fractions. Refer to
Table S1 in the
Supporting Information for the details of the peaks identified in the chromatogram; (
B) The major peak corresponds to the the active principle, identified as β-caryophyllene, isolated from the essential oil of
Aquilaria crassna.
Figure 2.
Gas chromatograpic analysis of
Aquilaria crassna and β-caryophyllene. (
A) Chemical characterization of the essential oil of
Aquilaria crassna by GC-MS. The pie charts depict the relative chemical composition of the sub-fractions. Refer to
Table S1 in the
Supporting Information for the details of the peaks identified in the chromatogram; (
B) The major peak corresponds to the the active principle, identified as β-caryophyllene, isolated from the essential oil of
Aquilaria crassna.
2.4. Antimicrobial Effect of β-Caryophyllene
β-Caryophyllene exhibited strong antibacterial effect against all the tested bacterial strains, with MIC values that ranged from 3 to 14 μM (
Table 2). The compound showed more pronounced antibacterial activity against Gram-positive bacteria than Gram-negative bacteria (
Table 2). The results revealed that
E. coli was less susceptible than the other Gram-positive bacterial strains tested. The lowest MIC value was recorded against S. aureus (3 ± 0.4 µM).
The antifungal assay results indicates a significant activity of β-caryophyllene against all the tested fungi (
Table 2). The activity of β-caryophyllene was more pronounced than the standard reference, kanamycin (
Table 2). The antimicrobial activity of β-caryophyllene could be attributed to its strong antioxidant activities [
21]. To the best of our knowledge, this is the first report on the antifungal activity of β-caryophyllene whereas, a previous study [
22] reported the significant antibacterial effect of β-caryophyllene against
S. aureus. In the present study, it is also demonstrated that β-caryophyllene significantly inhibits the growth of
S. aureus, whereas it is ineffective against
K. pneumoniae.
Figure 3 depicts the antibacterial (
Figure 3a) and antifungal (
Figure 3b) activities of β-caryophyllene against
S. aureus and
T. reesei, respectively.
Figure 3.
Antimicrobial effect of β-caryophyllene. (A) Antibacterial effect of β-caryophyllene (β-C) on Staphylococcus aureus (Std = Kanamycin; EOs = essential oil of Aquilaria crassna; NC = negative control); (B) Antifungal effect of β-caryophyllene (β-C) on Trichoderma reesei (Std = Kanamycin; NC = negative control).
Figure 3.
Antimicrobial effect of β-caryophyllene. (A) Antibacterial effect of β-caryophyllene (β-C) on Staphylococcus aureus (Std = Kanamycin; EOs = essential oil of Aquilaria crassna; NC = negative control); (B) Antifungal effect of β-caryophyllene (β-C) on Trichoderma reesei (Std = Kanamycin; NC = negative control).
Table 2.
Antimicrobial activity of β-caryophyllene.
Table 2.
Antimicrobial activity of β-caryophyllene.
| Bacterial Strains | MTCC | Minimum Inhibitory Concentration (MIC) in μM |
|---|
| β-Caryophyllene | Kanamycin |
|---|
| B. cereus | 1307 | 9 ± 1.1 | 2 ± 0.7 |
| B. subtilis | 6910 | 8 ± 2.1 | 4 ± 1.8 |
| S. aureus | 7405 | 3 ± 0.4 | 8 ± 2.3 |
| E. coli | 732 | 9 ± 2.2 | 7 ± 1.4 |
| K. pneumoniae | 7028 | 14 ± 2.7 | 2 ± 0.4 |
| P. aeruginosa | 4302 | 7 ± 1.2 | 9 ± 1.4 |
| A. niger | 2196 | 6 ± 0.8 | 7 ± 0.4 |
| P. citrinum | 7124 | 7 ± 1.2 | 9 ± 1.1 |
| R. oryzae | 1987 | 6 ± 0.5 | 7 ± 0.3 |
| T. reesei | 3929 | 4 ± 0.7 | 6 ± 1.4 |
2.5. Inhibitory Effect of β-Caryophyllene on the Proliferation of Cancer Cell Lines
The antiproliferative effect of β-caryophyllene was tested against seven tumor and two normal cell lines using the MTT assay. The median inhibitory concentration (IC
50) values were calculated for each cell line and the values are presented in
Table 3.
Table 3.
IC50 values of β-caryophyllene on various human cancer cell lines.
Table 3.
IC50 values of β-caryophyllene on various human cancer cell lines.
| Extracts | Carcinoma Cell Lines | Normal Cell Lines |
|---|
| HCT 116 | PANC-1 | HT-29 | ME-180 | PC3 | K562 | MCF-7 | CCD-18Co | NIH/3T3-L1 | RGC5 |
|---|
| A. crassna (µg/mL) | 28 | 32 | 82 | 98 | 79 | 142 | 110 | 579 | 133 | 98 |
| β-Caryophyllene (µM) | 19 | 27 | 63 | 95 | 104 | 105 | 285 | 612 | 530 | 156 |
| Positive controls (µM) | 5-FU a | BA b | 5-FU | BA | BA | BA | Tam c | BA | BA | BA |
| 12.7 | 19.4 | 15 | 37.1 | 8.4 | 17.3 | 9.5 | 62 | 53 | 97 |
Among the tested cancer cells, the compound demonstrated selective anti-proliferative effect against three cancer cell lines, namely HCT 116 (colon cancer, IC
50 = 19 µM), PANC-1 (pancreatic cancer, IC
50 = 27 µM), and HT29 (colon cancer, IC
50 = 63 µM) cells, whereas it exhibited either moderate or poor cytotoxic effects against ME-180, PC3, K562 and MCF-7. Noteworthily, the compound displayed low toxicity against the normal cell lines 3T3-L1 and RGC-5. The results were compared with the respective standard reference drugs, tamoxifen, betulinic acid and 5-fluorouracil.
Figure 4 shows the effect of β-caryophyllene on the morphological structures of the cancer cell lines. The photomicrographic images revealed that β-caryophyllene did not affect the morphology of a normal cell line (3T3-L1), as the treated cells were more or less similar to the negative control, even for the higher concentration of β-caryophyllene. The selectivity index (SI) measures the selective cytotoxicity of a test sample against cancerous cells and the safety of sample towards normal cells. Compounds with a SI value of more than 3 are considered to have high selectivity towards the particular cancer cell line [
23].
Table 4 presents the SI values of β-caryophyllene for various cancer cell lines tested. The analysis showed that β-caryophyllene possesses higher selectivity towards the colorectal cancer cells (HCT 116), with SI = 27.9, followed by PANC-1 and HT 29 cells with SI = 19.6 and 8, respectively. Therefore, in the present study further investigations on the cytotoxic effect of β-caryophyllene were carried out using HCT 116 cells.
Figure 5A shows a graphical illustration of the dose-dependent antiproliferative effect of the compound on the panel of human cancer cell lines.
Table 4.
Selectivity index of β-Caryophyllene which represents IC50 for normal cell line/IC50 for cancerous cell line.
Table 4.
Selectivity index of β-Caryophyllene which represents IC50 for normal cell line/IC50 for cancerous cell line.
| Extracts | Selectivity Index (SI) a |
|---|
| HCT 116 | PANC-1 | HT-29 | ME-180 | PC3 | K562 | MCF-7 |
|---|
| β-Caryophyllene (µM) | 32.2 | 22.7 | 9.7 | 6.4 | 5.9 | 5.8 | 2.1 |
2.6. β-Caryophyllene Reduces Mitochondrial Membrane Potential in HCT-116 Cells
In order to obtain a deeper insight into an apoptotic effect of β-caryophyllene on mitochondrial membrane potential, the rhodamine 123 assay was performed. In this assay, the lipophilic cationic dye rhodamine 123 was used to measure the mitochondrial function in the treated cells. Loss of membrane integrity and membrane potential (∆Ψ) in mitochondria of a cell are well known characteristic features of apoptosis [
24]. When the membrane potential in the mitochondria decreases, uptake of rhodamine 123 by the affected cells also decreases, which ultimately results in an exponential decrease in the fluorescence signal. Results of the present study revealed that the untreated cells displayed a strong fluorescence intensity indicating their unaffected health with good growth and cell proliferation (
Figure 5B). On the other hand, the fluorescence signal decreased remarkably in the cells treated with 10 μM β-caryophyllene (
Figure 5C,D), which suggests a reduced membrane potential due to the disrupted mitochondrial membrane. In addition, a time-dependent apoptotic effect of β-caryophyllene was recorded as the intensity of fluorescence signal dropped upon duration of the treatment (
Figure 5C,D). A similar effect can be seen in the cells treated with the standard drug 5-flourouracil (
Figure 5E). The apoptotic index estimated for β-caryophyllene treatment on HCT 116 cells after 24 h treatment was 64 ± 0.04 (
Figure 5F). The results can be compared in
Figure 5F with those of the standard reference 5-fluorouracil. The results provide additional information about the apoptotic properties of β-caryophyllene, and suggest that the apoptosis induced by β-caryophyllene in HCT 116 cells occurs via both the DNA and mitochondrial pathways.
Figure 4.
Effect of β-caryophyllene on the cellular morphology of human cancer and normal cell lines. Photomicrographic images of cancer cell lines, taken under an inverted phase-contrast microscope at 200× magnification using a digital camera at 48 h after treatment with β-caryophyllene.
Figure 4.
Effect of β-caryophyllene on the cellular morphology of human cancer and normal cell lines. Photomicrographic images of cancer cell lines, taken under an inverted phase-contrast microscope at 200× magnification using a digital camera at 48 h after treatment with β-caryophyllene.
Figure 5.
(A) Dose-dependent anti-proliferative effect of β-caryophyllene on, HCT 116, PANC-1, HT-29, MCF-7, PC3, K562, ME-180 and NIH/3T3-L1 cell lines was assessed by MTT-assay (values are represented as mean ± SD, n = 3); (B) Rhodamine 123 stained photomicrographic images of HCT 116 cells treated with vehicle (0.1% DMSO); (C) Rhodamine 123 stained photomicrographic images of HCT 116 cells treated with β-caryophyllene (10 µM) for 6 h; (D) Rhodamine 123 stained photomicrographic images of HCT 116 cells treated with β-caryophyllene (10 µM) for 12 h; (E) Rhodamine 123 stained photomicrographic images of HCT 116 cells treated with 5-flourouracil (10 µM) for 12 h; (F) Graphical representation of percentage of apoptotic indices. The apoptotic index for each test group was expressed as a percentage of the ratio of number of unstained cells to the total number of cell in 10 different microscopic fields. Values are presented as mean ± SD (n = 10), ** represents p < 0.01.
Figure 5.
(A) Dose-dependent anti-proliferative effect of β-caryophyllene on, HCT 116, PANC-1, HT-29, MCF-7, PC3, K562, ME-180 and NIH/3T3-L1 cell lines was assessed by MTT-assay (values are represented as mean ± SD, n = 3); (B) Rhodamine 123 stained photomicrographic images of HCT 116 cells treated with vehicle (0.1% DMSO); (C) Rhodamine 123 stained photomicrographic images of HCT 116 cells treated with β-caryophyllene (10 µM) for 6 h; (D) Rhodamine 123 stained photomicrographic images of HCT 116 cells treated with β-caryophyllene (10 µM) for 12 h; (E) Rhodamine 123 stained photomicrographic images of HCT 116 cells treated with 5-flourouracil (10 µM) for 12 h; (F) Graphical representation of percentage of apoptotic indices. The apoptotic index for each test group was expressed as a percentage of the ratio of number of unstained cells to the total number of cell in 10 different microscopic fields. Values are presented as mean ± SD (n = 10), ** represents p < 0.01.
2.7. β-Caryophyllene Induces Chromatin Condensation and DNA Fragmentation in HCT 116 Cells
HCT 116 cells were selected to study the effect of β-caryophyllene on nuclear changes and condensation using Hoechst 33342 stain. The vehicle-treated cells showed aggressively growing cells with prominent nuclei (
Figure 6A), whereas, the characteristic signs of apoptosis were noticed in the morphology of the nuclei of the β-caryophyllene-treated cells. β-Caryophyllene affected the nuclear morphology in a time-dependent manner (
Figure 6B,C). β-Caryophyllene at 10 μM concentration, caused significant nuclei condensation (arrows) after 6 h of treatment (
Figure 6B). This was clearly evidenced by lumping and squeezing of nuclear material to give irregularly distributed chromatin in the cytosol of the cells, whereas, at a later stage of treatment (after 12 h) the nuclear material is converted to shrunken and crescent-shaped structures (arrows), indicating the advanced features of apoptosis (
Figure 6C). At this stage, most of the treated cells showed discrete chromatin bodies suggesting the induction of karyorrhexis caused by β-caryophyllene. These results were compared with the standard drug, 5-fluorouracil (
Figure 6D). β-Caryophyllene displayed more pronounced effects compared to the standard reference drug. The apoptotic indices (
Figure 6E) for untreated HCT 116 cells were 1.7% ± 0.04%. However, following the treatment with β-caryophyllene for 24 h, the apoptotic indices against HCT 116 cells were significantly increased to 58.4% ± 7% (
Figure 6E).
It is well studied phenomenon that apoptotic cells go through a series of morphological changes namely, membrane blebbing, chromatin condensation, nuclear membrance disruption, nuclear fragmentation and dissolution [
25]. Recently, a study reported that β-caryophyllene induces apoptosis in a mouse blood cancer cell line through caspase-3 induction [
26]. Similarly, another study reported that a derivative of β-caryophyllene, β-caryophyllene oxide, induces apoptosis through suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation [
27]. In the present study, β-caryophyllene demonstrated clear signs of apoptosis in human colorectal carcinoma (HCT 116) cells.
In order to investigate the effect of β-caryophyllene on advanced stage of apoptosis, isolated DNA from β-caryophyllene-treated HCT 116 cells was analyzed by agarose gel electrophoresis. The result showed an obvious DNA fragmentation caused by β-caryophyllene as a dose-dependent laddering pattern was observed (
Figure 6F). The findings of the present study coincide with those of Amiel
et al. [
26], who reported a DNA fragmentation effect of β-caryophyllene in a mouse lymphoma cell line. However, for the first time, the present study demonstrated induction of nuclear condensation and fragmentation by β-caryophyllene in human cancer cells. The study of Amiel [
26] reported that β-caryophyllene activates the caspase-3 enzyme. Caspase-3 is an executioner enzyme in a caspase-dependent apoptosis cascade. Induction of caspase-3 activity in turn leads to chromatin condensation, degradation and dissolution. In the present study these facts were further confirmed and validated by Hoechst 33342 assay in β-caryophyllene-treated human colorectal cancer cells.
Figure 6.
Photomicrographs depicting images of HCT 116 cells with Hoechst 33258 staining (A) Cells treated with vehicle (0.1% DMSO). The vehicle-treated cells revealed an intact cell membrane with an evenly distributed nucleus in cytosol; (B) Cells after 6 h of β-caryophyllene (10 µM) treatment. Cells treated with β-caryophyllene displayed early stage apoptotic symptoms such as membrane blebbing and chromatin condensation (arrows); (C) Cells after 12 h of β-caryophyllene (10 µM) treatment. The arrows indicate the advanced staged apoptotic signs such as of nuclear dissolution including the half-moon (crescent) shaped apoptotic nuclei. In addition, at several places, the arrows mark chromatin breakdown and fragmentation; (D) Cells treated with standard reference, 5-flourouracil (10 µM) also exhibited significant induction of apoptosis in the cells; (E) Graphical representation of percentage of apoptotic indices for HCT 116 and PANC-1 cells. The apoptotic index for each test group was expressed as a percentage of the ratio of number of apoptotic cells to the total number of cell in 10 different microscopic fields. Values are presented as mean ± SD (n = 10), * represents p < 0.05 and ** represents p < 0.01; (F) Effect of β-caryophyllene (10 µM) on DNA fragmentation in HCT 116 cells after 24 h treatment. (i). The standard DNA ladder; (ii). The DNA fragmentation pattern of HCT 116 cells treated with 5-flourouracil (10 µM); (iii). DNA fragmentation pattern of HCT 116 cells treated with β-caryophyllene (5 µM); (iv). DNA fragmentation pattern of HCT 116 cells treated with β-caryophyllene (10 µM); (v). DNA fragmentation pattern of HCT 116 cells treated with 0.1% DMSO (negative control).
Figure 6.
Photomicrographs depicting images of HCT 116 cells with Hoechst 33258 staining (A) Cells treated with vehicle (0.1% DMSO). The vehicle-treated cells revealed an intact cell membrane with an evenly distributed nucleus in cytosol; (B) Cells after 6 h of β-caryophyllene (10 µM) treatment. Cells treated with β-caryophyllene displayed early stage apoptotic symptoms such as membrane blebbing and chromatin condensation (arrows); (C) Cells after 12 h of β-caryophyllene (10 µM) treatment. The arrows indicate the advanced staged apoptotic signs such as of nuclear dissolution including the half-moon (crescent) shaped apoptotic nuclei. In addition, at several places, the arrows mark chromatin breakdown and fragmentation; (D) Cells treated with standard reference, 5-flourouracil (10 µM) also exhibited significant induction of apoptosis in the cells; (E) Graphical representation of percentage of apoptotic indices for HCT 116 and PANC-1 cells. The apoptotic index for each test group was expressed as a percentage of the ratio of number of apoptotic cells to the total number of cell in 10 different microscopic fields. Values are presented as mean ± SD (n = 10), * represents p < 0.05 and ** represents p < 0.01; (F) Effect of β-caryophyllene (10 µM) on DNA fragmentation in HCT 116 cells after 24 h treatment. (i). The standard DNA ladder; (ii). The DNA fragmentation pattern of HCT 116 cells treated with 5-flourouracil (10 µM); (iii). DNA fragmentation pattern of HCT 116 cells treated with β-caryophyllene (5 µM); (iv). DNA fragmentation pattern of HCT 116 cells treated with β-caryophyllene (10 µM); (v). DNA fragmentation pattern of HCT 116 cells treated with 0.1% DMSO (negative control).
![Molecules 20 11808 g006]()
2.8. β-Caryophyllene Inhibits Motility and Invasion in HCT 116 Cells
In the vehicle (0.1% DMSO)-treated group, the cells were successfully migrated after 24 h (
Figure 7A,C), whereas β-caryophyllene exhibited a dose and time-dependent inhibitory effect on the motility of HCT 116 cells. The percentage of wound closure at sub-cytotoxic concentrations 5 (
Figure 7D,F) and 10 μM (
Figure 7G,I) was 66% ± 7% and 28% ± 2% inhibition, respectively at 24 h (
p < 0.01). The inhibitory effect of β-caryophyllene was more pronounced compared to that of the standard reference, 5-fluorouracil (
Figure 7J,L). At a concentration of 10 μM 5-fluorouracil produced 2 ± 1 and 34% ± 2% wound closure at 12 and 24 h, respectively (
p < 0.05) (
Figure 7M).
Figure 7.
Due to the successful migration of HCT 116 cells in the untreated group (negative control), the wound is almost closed after 24 h (A–C), whereas in the β-caryophyllene-treated monolayer, the wound remained open even after 24 h incubation. β-Caryophyllene (5 μM) caused a significant inhibition of HCT 116 cell migration (D–F). Interestingly, even at a sub-cytotoxic concentration (10 μM), the compound caused significant inhibition of migration (G–I). The results can be compared with those of the standard reference 5-FU (J–L). Graphical representation (M) of the time and dose and time-dependent inhibitory effect of β-caryophyllene on migration of HCT 116 (values are in mean ± SD, n = 6, * p < 0.1, ** p < 0.005).
Figure 7.
Due to the successful migration of HCT 116 cells in the untreated group (negative control), the wound is almost closed after 24 h (A–C), whereas in the β-caryophyllene-treated monolayer, the wound remained open even after 24 h incubation. β-Caryophyllene (5 μM) caused a significant inhibition of HCT 116 cell migration (D–F). Interestingly, even at a sub-cytotoxic concentration (10 μM), the compound caused significant inhibition of migration (G–I). The results can be compared with those of the standard reference 5-FU (J–L). Graphical representation (M) of the time and dose and time-dependent inhibitory effect of β-caryophyllene on migration of HCT 116 (values are in mean ± SD, n = 6, * p < 0.1, ** p < 0.005).
Photomicrographs (
Figure 8A) revealed the invasion of a large number of HCT 116 cells into the matrigel matrix, while β-caryophyllene (
Figure 8B,D) caused significant (
p < 0.05) inhibition. The total number of cells counted per microscopic field was 548 ± 79 for the negative control group. However, treatment with β-caryophyllene demonstrated significant (
p < 0.001) obstruction of the cell invasion, as the total number of invaded cells in treatment group was found to be drastically lower than the untreated group. The effect of β-caryophyllene was compared with the positive control 5-flourouracil (
Figure 8E). A dose dependent reduction was observed, as the concentrations 6.25, 12.5 and 25 μM, β-caryophyllene showed 258 ± 42, 118 ± 23 and 63 ± 12 invaded cells, respectively (
Figure 8F). Induction of apoptosis in cancer cells controls various rate limiting factors that obstruct tumourigenesis and metastasis. Tumour cells easily detach from the tumour tissue and escape the primary site. The tumour cells enter the blood circulation, wherein they continue their proliferation and multiplication and localize into the secondary site. Metastasis occurs basically by the virtue of migratory and invasive cascades of the cancer cells. The ability of motility and invasion in cancerous cells is a prerequisite feature which promotes tumourigenesis and metastasis in body. Inhibition of
in vitro migration of HCT 116 cells by β-caryophyllene indicates that it has a potential to suppress the motility of cancer cells and thus it could control metastatic propagation of malignancy [
28]. Similarly, β-caryophyllene considerably restricted invasion of HCT 116 through the matrigel basement which strongly supports that notion that β-caryophyllene has an ability to interrupt cell invasion, a basic characteristic of metastatic cascade [
29]. These findings support the anti-metastatic potential of β-caryophyllene against colorectal cancer.
Figure 8.
(A) Photomicrographs of HCT 116 cells invading the matrigel barrier. The negative control group showed a large number of invaded cells; (B‒D) Photomicrographs of HCT 116 cells showing the anti-invasion effect of β-caryophyllene (6.25, 12.5 and 25 µM, respectively) on a matrigel matrix; (E) Photomicrographic images of HCT 116 cells showing the anti-invasion effect of 5-flourouracil (10 µM) on a matrigel matrix; (F) Graphical representation of mean number of cells invaded per field of view, after counting 10 microscopic fields of view for triplicate wells. In untreated wells, the population of the cells invaded through the matrigel was significantly more than that of the treated wells (*p < 0.05, ** p < 0.01); (G) Effect of β-caryophyllene on survival of HCT 116 colonies in a colony formation assay. The picture clearly depicts the strong anti-clonogenic effect of β-caryophyllene on colonies of the cancer cells; (H) The graphical representation illustrates the percentage of plating efficiencies after the treatment of the cells with β-caryophyllene in comparison with negative control and the standard reference drug, 5-flourouracil. The results were presented as mean ± SD, n = 3.
Figure 8.
(A) Photomicrographs of HCT 116 cells invading the matrigel barrier. The negative control group showed a large number of invaded cells; (B‒D) Photomicrographs of HCT 116 cells showing the anti-invasion effect of β-caryophyllene (6.25, 12.5 and 25 µM, respectively) on a matrigel matrix; (E) Photomicrographic images of HCT 116 cells showing the anti-invasion effect of 5-flourouracil (10 µM) on a matrigel matrix; (F) Graphical representation of mean number of cells invaded per field of view, after counting 10 microscopic fields of view for triplicate wells. In untreated wells, the population of the cells invaded through the matrigel was significantly more than that of the treated wells (*p < 0.05, ** p < 0.01); (G) Effect of β-caryophyllene on survival of HCT 116 colonies in a colony formation assay. The picture clearly depicts the strong anti-clonogenic effect of β-caryophyllene on colonies of the cancer cells; (H) The graphical representation illustrates the percentage of plating efficiencies after the treatment of the cells with β-caryophyllene in comparison with negative control and the standard reference drug, 5-flourouracil. The results were presented as mean ± SD, n = 3.
![Molecules 20 11808 g008]()
2.9. β-Caryophyllene Inhibits Clonogenicity and the Growth of Tumor Spheroids
Colonization of cancer cells is one of the essential criteria for tumorigenesis.
Figure 8G clearly shows the dose-dependent inhibitory effect of β-caryophyllene on colony formation of HCT 116 cells. The results showed significant suppression of colonization of the cancer cells. Percentage of plating efficiency (PE) in negative control (untreated cells) group was 78.4% ± 3%, which was drastically decreased upon treatment with β-caryophyllene. At concentrations of 5 and 10 µM, β-caryophyllene showed potent cytotoxic effects against the cancer cells as the percentage of plating efficiency recorded was 43.6% ± 4% and 35.2% ± 5%, respectively (
Figure 8H). The graphical representation (
Figure 8H) illustrates the quantitative estimation of the efficacy of β-caryophyllene against the clonogenicity of colorectal cancer cells. The results of colony formation assay suggested that β-caryophyllene can significantly stop colonization of HCT 116 cells.
Figure 9.
Anti-tumor aggregation effects of β-caryophyllene on in vitro HCT 116 cellular spheroids in a hanging drop assay. The cellular aggregates treated with vehicle (0.1% DMSO) developed in a solid spheroid shape within 9 days (A–C), whereas, β-caryophyllene at a concentration of 5 µM displayed significant inhibitory effect on the HCT 116 cellular aggregate microspheroids (D–F). At a concentration 10 µM, β-caryophyllene completely obliterated the solid cellular aggregates of HCT 116 cells on the 9th day of seeding (G–I). Similar effects were observed with the standard reference, 5-flourouracil (J–L). Graphical representation (M) of the dose dependent inhibitory effect of β-caryophyllene on in vitro cellular aggregates of HCT 116 cells on 9th day of treatment. The results are presented as mean ± SD, n = 6 (* p < 0.05, ** p < 0.01).
Figure 9.
Anti-tumor aggregation effects of β-caryophyllene on in vitro HCT 116 cellular spheroids in a hanging drop assay. The cellular aggregates treated with vehicle (0.1% DMSO) developed in a solid spheroid shape within 9 days (A–C), whereas, β-caryophyllene at a concentration of 5 µM displayed significant inhibitory effect on the HCT 116 cellular aggregate microspheroids (D–F). At a concentration 10 µM, β-caryophyllene completely obliterated the solid cellular aggregates of HCT 116 cells on the 9th day of seeding (G–I). Similar effects were observed with the standard reference, 5-flourouracil (J–L). Graphical representation (M) of the dose dependent inhibitory effect of β-caryophyllene on in vitro cellular aggregates of HCT 116 cells on 9th day of treatment. The results are presented as mean ± SD, n = 6 (* p < 0.05, ** p < 0.01).
In a hanging drop environment, tumour cells aggregate and sediment in the cultures due to the applied gravitational force on the suspended cells. In the hanging drop assay, the cells were allowed to sediment and aggregate and later the cellular aggregates were harvested and seeded onto an agar-coated plate. The cellular aggregates from the hanging drop adopt a three-dimensional spheroid shape on the agar plate which leads to transformation of suspended cells into cellular aggregates which ultimately from a solid spheroid shape within the first 24 h (negative control,
Figure 9A,C).
Figure 9D,F illustrate the inhibitory effect of β-caryophyllene (5 µM) on the growth of aggregates and spheroids of HCT 116 cells. A stronger inhibitory effect of β-caryophyllene (10 µM) on tumour spheroids was recorded and is depicted in
Figure 9G,I.
Initially, the cellular aggregates were prepared from 20 µL hanging drops (5000 cells/drop) which later on developed into a thick and dense tumor-shaped spheroid within 24 h as shown in the negative control group (
Figure 9A,C). β-Caryophyllene displayed a dose-dependent inhibitory effect on the HCT 116 tumour spheroids. At concentrations of 5 and 10 µM, β-caryophyllene produced significant inhibitory effects of 23% ± 4% and 68% ± 8% on the density of the tumour spheroids, respectively (
Figure 9D,I). The results are compared with that of the standard reference, 5-Fluorouracil which showed 81% ± 4% inhibition at 10 µM concentration (
Figure 9J,L).
Figure 9M illustrates the quantitative data of dose-dependent effect of β-caryophyllene in comparison with 5-fluorouracil. The inhibition of cellular aggregation is directly associated with the significant anti-proliferation effect on HCT 116 cells. The MTT assay in the present study supports the findings of the tumour spheroid assay.