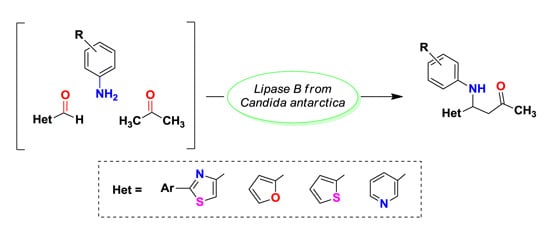
3.3. Enzymatic Synthesis of Heterocyclic Mannich Bases
3.3.1. Lipase Screening for the Enzymatic Mannich Reaction
To a solution of 2-phenylthiazol-4-carbaldehyde 1a (14.16 mg, 75 μmol) dissolved in acetone (600 μL), aniline (7.5 μL, 82.5 μmol), deionized water (600 μL) and different lipases (15 mg) were added in this order. The reaction mixtures were shaken at 500 rpm for 20 h and then analyzed by TLC using a mixture of petroleum ether: ethyl acetate 4:1 (v/v) as eluent. The enzyme was removed by centrifugation and filtration and then it was washed three times with acetone in order to recuperate the product. The filtrate was brought to dryness by rotatory evaporation under reduced pressure. The crude product was purified by column chromatography, using a mixture of petroleum ether: ethyl acetate 4:1 (v/v). The purified product was dried and weighed.
3.3.2. Analytical Scale CaL-B Catalyzed Direct Mannich Reaction Using Different Aldehydes and Aromatic Amines as Substrates
To a solution of 2-arylthiazol-4-carbaldehyde 1a–d (25 μmol) dissolved in acetone (200 μL), aromatic primary amine (27.5 μmol of aniline sulfate/p-toluidine/4-chloroaniline/4-nitroaniline), deionized water (200 μL) and CaL-B (4 mg) were added in this order. In the case when aniline sulfate was used as substrate, sodium acetate (2.26 mg, 27.5 μmol) was added to the reaction mixture. The reaction mixtures were shaken at 500 rpm for 20 h and then analyzed by TLC using a mixture of petroleum ether: ethyl acetate 4:1 (v/v) as eluent.
3.3.3. Preparative Scale Enzymatic Synthesis of Thiazolic Mannich Bases 4a–k
To a solution of heterocyclic aldehyde 1a–g (1.5 mmol of 2-arylthiazole-4-carbaldehyde, furane-2-carbaldehyde, thiophene-2-carbaldehyde, pyridine-3-carbaldehyde) dissolved in acetone (10 mL), aromatic primary amine (1.65 mmol of aniline sulphate/p-toluidine), deionized water (10 mL) and CaL-B (150 mg) were added in this order. When aniline sulphate was used as substrate, sodium acetate (136 mg, 1.65 mmol) was added to the reaction mixture. The reaction mixtures were shaken at 1200 rpm until complete consumption of aldehydes 1a–g (2–3 days, checked by TLC using a mixture of petroleum: ethyl acetate 4:1 (v/v) as eluent). After completion of the reaction, the enzyme was removed by filtration and washed three times with acetone in order to recuperate the product. The filtrate was concentrated under reduced pressure, in order to remove the acetone. The aqueous phase was extracted three times with dichloromethane. The organic phase was dried over anhydrous Na2SO4 and the solvent was evaporated under reduced pressure. The crude product was purified by column chromatography, using as eluent a mixture of petroleum ether: ethyl acetate 4:1 (v/v) for compounds 4a–h, dichloromethane for compounds 4i,j and respectively a mixture of dichloromethane:acetone 2:1 (v/v) for compound 4k. The obtained Mannich bases were stored in sealed flasks, at 0 °C.
4-(Phenylamino)-4-(2-phenylthiazol-4-yl)butan-2-one (4a) white solid, yield: 75%, mp 117 °C. 1H-NMR (300 MHz, CDCl3) δ 7.95–7.92 (m, 2H), 7.47–7.43 (m, 3H), 7.20–7.13 (m, 3H), 6.76–6.69 (m, 3H), 5.13 (t, J = 5.8 Hz, 1H), 4.62 (s, 1H), 3.17 (d, J = 6.1 Hz, 2H), 2.16 (s, 3H). 13C-NMR (75 MHz, CDCl3) δ 207.76, 168.52, 158.10, 146.72, 133.72, 130.17, 129.44, 129.07, 126.57, 118.28, 114.96, 113.98, 51.20, 48.35, 31.02. GC-MS: m/z found: 322 (M calculated for C19H18N2OS: 322); m/z (%) = 323 (M + 1, 3), 322 (M, 13), 279 (18), 266 (20), 265 (100), 188 (26), 162 (10), 121 (10), 104 (15), 93 (8), 77 (33), 65 (13), 43 (90), 39 (6).
4-(Phenylamino)-4-(2-m-tolylthiazol-4-yl)butan-2-one (4b) oily liquid, yield: 70%, 1H-NMR (600 MHz, CDCl3) δ 7.77 (s, 1H), 7.72 (d, J = 7.8 Hz, 1H), 7.33 (t, J = 7.6 Hz, 1H), 7.24 (d, J = 7.6 Hz, 1H), 7.18 (t, J = 7.9 Hz, 2H), 7.11 (s, 1H), 6.74 (t, J = 7.3 Hz, 1H), 6.69 (d, J = 7.8 Hz, 2H), 5.14 (t, J = 6.1 Hz, 1H), 3.17 (d, J = 6.2 Hz, 2H), 2.43 (s, 3H), 2.16 (s, 3H). 13C-NMR (151 MHz, CDCl3) δ 207.68, 168.72, 157.96, 146.69, 138.76, 133.56, 130.93, 129.36, 128.91, 127.04, 123.75, 118.19, 114.77, 113.93, 51.17, 48.33, 30.90, 21.43. GC-MS: m/z found: 336 (M calculated for C20H20N2OS: 336); m/z (%) = 336 (M, 3), 293 (3), 279 (15), 243 (40), 228 (89), 214 (26), 200 (47), 186 (13), 160 (9), 149 (100), 135 (16), 116 (10), 93 (43), 83 (17), 77 (23), 65 (24), 43 (70), 39 (58), 27 (9).
4-(Phenylamino)-4-(2-p-tolylthiazol-4-yl)butan-2-one (4c) oily liquid, yield: 68%, 1H-NMR (400 MHz, CDCl3) δ 7.82 (d, J = 8.1 Hz, 2H), 7.24 (d, J = 8.5 Hz, 2H), 7.19–7.15 (m, 2H), 7.08 (s, 1H), 6.73 (t, J = 7.3 Hz, 1H), 6.69 (d, J = 7.7 Hz, 2H), 5.13 (t, J = 6.1 Hz, 1H), 3.16 (d, J = 6.1 Hz, 2H), 2.39 (s, 3H), 2.15 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 207.67, 168.62, 157.85, 146.70, 134.82, 131.05, 129.66, 129.37, 126.43, 118.18, 114.36, 113.94, 51.18, 48.32, 30.92, 21.48. GC-MS: m/z found: 336 (M calculated for C20H20N2OS: 336); m/z (%) = 336 (M, 7), 293 (8), 279 (42), 243 (48), 228 (93), 215 (6), 200 (70), 135 (13), 116 (16), 93 (44), 83 (20), 77 (17), 65 (24), 43 (100), 39 (66), 27 (3).
4-(2-(4-Bromophenyl)thiazol-4-yl)-4-(phenylamino)butan-2-one (4d) semisolid, yield: 66%, 1H-NMR (600 MHz, CDCl3) δ 7.79 (d, J = 8.5 Hz, 2H), 7.56 (d, J = 8.5 Hz, 2H), 7.18 (t, J = 7.9 Hz, 2H), 7.14 (s, 1H), 6.74 (t, J = 7.3 Hz, 1H), 6.69 (d, J = 7.8 Hz, 2H), 5.14 (t, J = 6.1 Hz, 1H), 3.16 (d, J = 6.2 Hz, 2H), 2.16 (s, 3H). 13C-NMR (151 MHz, CDCl3) δ 207.43, 167.03, 158.34, 146.59, 132.52, 132.10, 129.37, 127.87, 124.26, 118.25, 115.23, 113.89, 51.02, 48.24, 30.84. GC-MS: m/z found: 400 (M calculated for C19H17BrN2OS: 400); m/z (%) = 402 (M, 1, 81Br), 400 (M, 1, 79Br), 359 (1, 81Br), 357 (1, 79Br), 345 (2, 81Br), 343 (2, 79Br), 309 (18, 81Br), 307 (18, 79Br), 294 (35, 81Br), 292 (34, 79Br), 266 (13, 81Br), 264 (11, 79Br), 149 (20), 93 (17), 83 (18), 43 (100), 39 (27), 27 (6).
4-(p-Tolylamino)-4-(2-phenylthiazol-4-yl)butan-2-one (4e) oily liquid, yield: 72%, 1H-NMR (600 MHz, CDCl3) δ 7.96–7.94 (m, 2H), 7.46–7.43 (m, 3H), 7.13 (s, 1H), 7.00 (d, J = 8.2 Hz, 2H), 6.63 (d, J = 8.4 Hz, 2H), 5.12 (t, J = 6.1 Hz, 1H), 3.16 (d, J = 6.2 Hz, 2H), 2.25 (s, 3H), 2.16 (s, 3H). 13C-NMR (151 MHz, CDCl3) δ 207.66, 168.34, 158.26, 144.37, 134.72, 133.67, 130.67, 130.06, 129.86, 128.97, 126.48, 115.29, 51.52, 48.35, 30.86, 20.46. GC-MS: m/z found: 336 (M calculated for C20H20N2OS: 336); m/z (%) = 336 (M, 6), 293 (4), 279 (29), 243 (3), 229 (46), 214 (100), 201 (7), 186 (39), 160 (13), 106 (21), 91 (11), 83 (37), 77 (35), 65 (11), 43 (24), 39 (73), 27 (5).
4-(p-Tolylamino)-4-(2-m-tolylthiazol-4-yl)butan-2-one (4f) oily liquid, yield: 68%, 1H-NMR (600 MHz, CDCl3) δ 7.76 (s, 1H), 7.72 (d, J = 7.7 Hz, 1H), 7.32 (t, J = 7.6 Hz, 1H), 7.24 (d, J = 7.6 Hz, 1H), 7.11 (s, 1H), 6.98 (d, J = 8.2 Hz, 2H), 6.62 (d, J = 8.4 Hz, 2H), 5.10 (t, J = 6.2 Hz, 1H), 3.15 (d, J = 6.2 Hz, 2H), 2.43 (s, 3H), 2.23 (s, 3H), 2.16 (s, 3H). 13C-NMR (151 MHz, CDCl3) δ 207.73, 168.66, 158.18, 144.39, 138.76, 134.79, 133.61, 130.90, 129.86, 128.90, 127.05, 123.76, 114.73, 114.23, 51.59, 48.42, 30.88, 21.44, 20.47. GC-MS: m/z found: 350 (M calculated for C21H22N2OS: 350); m/z (%) = 350 (M, 6), 293 (23), 243 (57), 228 (100), 215 (7), 200 (46), 135 (7), 91 (18), 83 (25), 65 (12), 43 (33), 39 (20).
4-(p-Tolylamino)-4-(2-p-tolylthiazol-4-yl)butan-2-one (4g) oily liquid, yield: 69%, 1H-NMR (600 MHz, CDCl3) δ 7.84 (d, J = 8.0 Hz, 2H), 7.25 (d, J = 8.0 Hz, 2H), 7.09 (s, 1H), 7.00 (d, J = 8.2 Hz, 2H), 6.64 (d, J = 8.3 Hz, 2H), 5.12 (t, J = 6.2 Hz, 1H), 3.16 (d, J = 6.2 Hz, 2H), 2.41 (s, 3H), 2.25 (s, 3H), 2.16 (s, 3H). 13C-NMR (151 MHz, CDCl3) δ 207.65, 168.49, 157.99, 144.32, 140.21, 130.99, 129.78, 129.58, 127.35, 126.36, 114.28, 114.16, 51.49, 48.31, 30.77, 21.39, 20.40. GC-MS: m/z found: 350 (M calculated for C21H22N2OS: 350); m/z (%) = 350 (M, 7), 293 (25), 243 (56), 228 (100), 215 (6), 200 (44), 135 (6), 91 (15), 83 (24), 65 (14), 43 (35), 39 (18).
4-(p-Tolylamino)-4-(2-(4-bromophenyl)thiazol-4-yl)butan-2-one (4h) white solid, yield: 66%, mp: 123–124 °C. 1H-NMR (400 MHz, CDCl3) δ 7.80 (d, J = 8.5 Hz, 2H), 7.56 (d, J = 8.5 Hz, 2H), 7.15 (s, 1H), 7.00 (d, J = 8.2 Hz, 2H), 6.63 (d, J = 8.2 Hz, 2H), 5.11 (t, J = 6.1 Hz, 1H), 3.16 (d, J = 6.1 Hz, 2H), 2.24 (s, 3H), 2.16 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 207.43, 166.94, 158.49, 144.20, 132.55, 132.08, 129.85, 127.86, 127.57, 124.22, 115.21, 114.22, 51.46, 48.28, 30.80, 20.45. GC-MS: m/z found: 414 (M calculated for C20H19BrN2OS: 414); m/z (%) = 416 (M, 2, 81Br), 414 (M, 2, 79Br), 359 (10, 81Br), 357 (10, 79Br), 309 (4, 81Br), 307 (4, 79Br), 294 (9, 81Br), 292 (9, 79Br), 268 (6, 81Br), 266 (8, 79Br), 191 (13), 149 (52), 106 (100), 84 (8), 77 (14), 43 (35), 27 (7).
4-(Furan-2-yl)-4-(phenylamino)butan-2-one (4i) yellow solid, mp: 69 °C, yield: 74%. 1H-NMR (600 MHz, CDCl3) δ 7.34 (s, 1H), 7.18 (t, J = 7.9 Hz, 2H), 6.75 (t, J = 7.3 Hz, 1H), 6.68 (d, J = 8.4 Hz, 2H), 6.29 (dd, J = 3.1, 1.9 Hz, 1H), 6.19 (d, J = 3.2 Hz, 1H), 5.04 (t, J = 6.3 Hz, 1H), 3.05 (dd, J = 16.4, 6.4 Hz, 1H), 3.00 (dd, J = 16.4, 6.4 Hz, 1H), 2.14 (s, 3H). 13C-NMR (151 MHz, CDCl3) δ 206.78, 154.74, 146.53, 141.70, 129.31, 118.42, 113.96, 110.42, 106.41, 48.32, 47.23, 30.67. GC-MS: m/z found: 229 (M calculated for C14H15NO2: 229); m/z (%) = 229 (M, 1); 143 (1); 134 (2); 92 (2); 77 (3); 65 (6); 43 (100); 39 (12); 27 (7).
4-(Phenylamino)-4-(thiophen-2-yl)butan-2-one (4j) yellow solid, mp: 70 °C, yield: 76%. 1H-NMR (600 MHz, CDCl3) δ 7.21–7.10 (m, 3H), 6.96 (d, J = 3.5 Hz, 1H), 6.92 (dd, J = 5.0, 3.5 Hz, 1H), 6.73 (t, J = 7.3 Hz, 1H), 6.65 (d, J = 7.7 Hz, 2H), 5.18 (t, J = 6.2 Hz, 1H), 3.08 (dd, J = 16.4, 6.3 Hz, 1H), 3.02 (dd, J = 16.4, 6.3 Hz, 1H), 2.15 (s, 3H). 13C-NMR (151 MHz, CDCl3) δ 206.75, 147.32, 146.56, 129.39, 127.04, 124.32, 124.09, 118.62, 114.16, 50.90, 50.48, 31.03. GC-MS: m/z found: 245 (M calculated for C14H15NOS: 245); m/z (%) = 245 (M, 1); 188 (2); 139 (6); 118 (4); 92 (7); 77 (2); 43 (100); 39 (6); 27 (4).
4-(Phenylamino)-4-(pyridin-3-yl)butan-2-one (4k) white solid, mp: 125 °C, yield: 80%. 1H-NMR (600 MHz, CDCl3) δ 8.64 (d, J = 2.0 Hz, 1H), 8.48 (dd, J = 4.7, 1.4 Hz, 1H), 7.70 (dd, J = 6.3, 1.6 Hz, 1H), 7.22 (dd, J = 7.8, 4.8 Hz, 1H), 7.09 (t, J = 7.9 Hz, 2H), 6.68 (t, J = 7.3 Hz, 1H), 6.53 (d, J = 7.8 Hz, 2H), 4.89 (t, J = 6.3 Hz, 1H), 2.96 (dd, J = 6.3, 1.9 Hz, 2H), 2.12 (s, 3H). 13C-NMR (151 MHz, CDCl3) δ 206.44, 148.78, 148.47, 146.35, 138.13, 134.34, 129.33, 123.76, 118.37, 113.88, 52.01, 50.66, 30.75. GC-MS: m/z found: 240 (M calculated for C15H16N2O: 240); m/z (%) = 240 (M, 22); 206 (13); 191 (53); 183 (100); 167 (7); 149 (28); 146 (50); 132 (69); 123 (14); 118 (10); 106 (23); 104 (58); 93 (21); 77 (38); 43 (55); 39 (15); 27 (7).
3.4. Synthesis of N-Acetylated Mannich Bases 5a–k
To a solution of Mannich base 4a–k (0.6 mmol) in anhydrous dichloromethane (4 mL), acetic anhydride (3 mmol) and 1 drop of pyridine were added. The reaction mixture was stirred overnight. After completion of the reaction (checked by TLC using dichloromethane as eluent), the reaction mixture was quenched with water. The organic layer was dried over anhydrous Na2SO4 and the solvent was evaporated under reduced pressure. The crude product was purified by column chromatography on silicagel, in a gradient separation procedure, using a mixture of dichloromethane: acetone from 25:1 to 9:1 (v/v) for compounds 5a–j and respectively a mixture of dichloromethane:acetone 9:2 v/v for compound 5k.
N-Acetyl-4-(phenylamino)-4-(2-phenylthiazol-4-yl)butan-2-one (5a): yellow liquid, yield: 81%, 1H-NMR (600 MHz, CDCl3) δ 7.90–7.77 (m, 2H), 7.46–7.36 (m, 3H), 7.35–7.28 (m, 3H), 7.22 (s, 1H), 7.15–6.88 (m, 2H), 6.46 (t, J = 7.5 Hz, 1H), 3.10 (ddd, J = 24.6, 16.6, 7.5 Hz, 2H), 2.13 (s, 3H), 1.79 (s, 3H). 13C-NMR (151 MHz, CDCl3) δ 205.89, 170.59, 167.23, 155.74, 140.24, 133.67, 130.05, 129.94, 129.29, 128.97, 128.43, 126.52, 116.90, 51.62, 45.81, 30.28, 23.60. GC-MS: m/z found: 364 (M calculated for C21H20N2O2S: 364); m/z (%) = 365 (M + 1, 2.7), 364 (M, 8.2), 321 (82.2), 279 (100), 265 (30.0), 188 (33.7), 93 (28.3), 77 (10.4), 43 (23.9), 39 (8.4).
N-Acetyl-4-(phenylamino)-4-(2-m-tolylthiazol-4-yl)butan-2-one (5b): yellow liquid, yield: 81%, 1H-NMR (600 MHz, CDCl3) δ 7.66 (s, 1H), 7.51 (d, J = 7.9 Hz, 1H), 7.36–7.27 (m, 4H), 7.22 (d, J = 7.5 Hz, 1H), 7.19 (s, 1H), 7.13–6.98 (m, 2H), 6.47 (t, J = 7.4 Hz, 1H), 3.10 (ddd, J = 24.6, 16.5, 7.5 Hz, 2H), 2.40 (s, 3H), 2.14 (s, 3H), 1.80 (s, 3H). 13C-NMR (151 MHz, CDCl3) δ 206.02, 170.69, 167.56, 155.65, 138.78, 133.65, 130.92, 130.01, 129.33, 128.92, 128.47, 127.19, 123.78, 119.96, 116.83, 51.74, 45.89, 30.30, 23.64, 21.51. GC-MS: m/z found: 378 (M calculated for C22H22N2O2S: 378); m/z (%) = 379 (M + 1, 0.2), 378 (M, 1), 335 (14), 293 (24), 279 (6), 202 (11), 149 (2), 135 (5), 118 (8), 93 (12), 77 (10), 43 (100), 39 (10).
N-Acetyl-4-(phenylamino)-4-(2-p-tolylthiazol-4-yl)butan-2-one (5c): yellow liquid, yield: 80%, 1H-NMR (600 MHz, CDCl3) δ 7.74 (d, J = 8.1 Hz, 2H), 7.32 (s, 3H), 7.21 (d, J = 8.0 Hz, 2H), 7.17 (s, 1H), 7.13–6.94 (m, J = 23.7, 16.5, 11.5 Hz, 2H), 6.45 (t, J = 7.5 Hz, 1H), 3.10 (ddd, J = 24.6, 16.5, 7.5 Hz, 2H), 2.38 (s, 3H), 2.14 (s, 3H), 1.80 (s, 3H). 13C-NMR (151 MHz, CDCl3) δ 206.00, 170.62, 167.47, 155.54, 140.36, 131.08, 129.99, 129.67, 129.30, 128.43, 126.50, 119.91, 116.42, 51.73, 45.87, 30.31, 23.64, 21.54. GC-MS: m/z found: 378 (M calculated for C22H22N2O2S: 378); m/z (%) = 378 (M, 1), 335 (6), 293 (13), 279 (4), 202 (13), 149 (22), 135 (4), 118 (3), 93 (11), 77 (5), 43 (100), 39 (16).
N-Acetyl-4-(2-(4-bromophenyl)thiazol-4-yl)-4-(phenylamino)butan-2-one (5d): white solid, mp: 127 °C, yield: 78%, 1H-NMR (600 MHz, CDCl3) δ 7.71 (d, J = 8.4 Hz, 2H), 7.54 (d, J = 8.5 Hz, 2H), 7.39–7.29 (m, 3H), 7.25 (s, 1H), 7.16–6.88 (m, 2H), 6.43 (t, J = 7.4 Hz, 1H), 3.11 (ddd, J = 24.5, 16.7, 7.5 Hz, 2H), 2.14 (s, 3H), 1.80 (s, 3H). 13C-NMR (151 MHz, CDCl3) δ 205.89, 170.73, 166.04, 156.04, 140.32, 132.22, 129.96, 129.38, 128.54, 128.01, 124.37, 122.41, 117.32, 51.71, 45.88, 30.33, 23.64. GC-MS: m/z found: 442 (M calculated for C21H19BrN2O2S: 442); m/z (%) = 444 (M, 1, 81Br), 442 (M, 1, 79Br), 401 (5, 81Br), 399 (5, 79Br), 359 (6, 81Br), 357 (6, 79Br), 345 (2, 81Br), 343 (2, 79Br), 310 (1, 81Br), 308 (1, 79Br), 149 (4), 93 (18), 77 (15), 43 (100), 39 (12), 27 (2).
N-Acetyl-4-(p-tolylamino)-4-(2-phenylthiazol-4-yl)butan-2-one (5e): yellow liquid, yield: 76%, 1H-NMR (600 MHz, CDCl3) δ 7.94–7.79 (m, J = 6.5, 2.7 Hz, 2H), 7.46–7.35 (m, J = 4.9 Hz, 3H), 7.21 (s, 1H), 7.16–7.05 (m, 2H), 7.02–6.75 (m, 2H), 6.45 (t, J = 7.5 Hz, 1H), 3.08 (ddd, J = 24.6, 16.6, 7.5 Hz, 2H), 2.33 (s, 3H), 2.14 (s, 3H), 1.79 (s, 3H). 13C-NMR (151 MHz, CDCl3) δ 205.97, 170.81, 167.17, 155.86, 138.35, 137.50, 133.71, 130.04, 129.92, 129.63, 128.96, 126.54, 116.88, 51.47, 45.81, 30.30, 23.55, 21.19. GC-MS: m/z found: 378 (M calculated for C22H22N2O2S: 378); m/z (%) = 379 (M + 1, 3), 378 (M, 2), 335 (9), 293 (10), 279 (4), 230 (4), 188 (10), 149 (4), 91 (4), 77 (7), 65 (5), 43 (100), 39 (8).
N-Acetyl-4-(p-tolylamino)-4-(2-m-tolylthiazol-4-yl)butan-2-one (5f): yellow liquid, yield: 75%, 1H-NMR (600 MHz, CDCl3) δ 7.65 (s, 1H), 7.38 (d, J = 8.3 Hz, 1H), 7.29 (t, J = 7.6 Hz, 1H), 7.22 (d, J = 7.5 Hz, 1H), 7.18 (s, 1H), 7.11–7.08 (m, J = 8.2 Hz, 2H), 7.01–6.71 (m, J = 46.4 Hz, 2H), 6.46 (t, J = 7.5 Hz, 1H), 3.09 (ddd, J = 24.6, 16.5, 7.5 Hz, 2H), 2.40 (s, 3H), 2.34 (s, 3H), 2.14 (s, 3H), 1.80 (s, 3H). 13C-NMR (151 MHz, CDCl3) δ 206.07, 170.90, 167.51, 155.67, 138.75, 133.88, 130.91, 129.93, 129.67, 129.53, 128.90, 127.19, 123.80, 120.11, 116.79, 51.55, 45.86, 30.28, 23.55, 21.49, 21.20. GC-MS: m/z found: 392 (M calculated for C23H24N2O2S: 392); m/z (%) = 393 (M + 1, 1), 392 (M, 2), 349 (14), 307 (14), 293 (6), 243 (5), 228 (13), 202 (3), 149 (21), 107 (28), 91 (9), 77 (3), 65 (9), 43 (100), 39 (22).
N-Acetyl-4-(p-tolylamino)-4-(2-p-tolylthiazol-4-yl)butan-2-one (5g): yellow liquid, yield: 76%, 1H-NMR (600 MHz, CDCl3) δ 7.73 (d, J = 8.0 Hz, 2H), 7.19 (d, J = 7.7 Hz, 2H), 7.14 (s, 1H), 7.12–6.74 (m, 4H), 6.45 (t, J = 7.5 Hz, 1H), 3.06 (ddd, J = 24.6, 16.5, 7.5 Hz, 2H), 2.36 (s, 3H), 2.32 (s, 3H), 2.13 (s, 3H), 1.78 (s, 3H). 13C-NMR (151 MHz, CDCl3) δ 205.96, 170.71, 167.29, 155.63, 140.21, 138.26, 137.46, 131.08, 129.84, 129.61, 129.58, 126.42, 116.29, 51.44, 45.77, 30.23, 23.49, 21.45, 21.13. GC-MS: m/z found: 392 (M calculated for C23H24N2O2S: 392); m/z (%) = 393 (M + 1, 1), 392 (M, 4), 349 (24), 307 (29), 293 (7), 243 (27), 228 (44), 202 (30), 149 (36), 135 (7), 118 (8), 107 (62), 91 (21), 77 (19), 65 (14), 43 (100), 39 (27).
N-Acetyl-4-(p-tolylamino)-4-(2-(4-bromophenyl)thiazol-4-yl)butan-2-one (5h): semisolid, yield: 80%, 1H-NMR (600 MHz, CDCl3) δ 7.67 (d, J = 8.4 Hz, 2H), 7.48 (d, J = 8.5 Hz, 2H), 7.21 (s, 1H), 7.11–6.73 (m, J = 139.3 Hz, 4H), 6.41 (t, J = 7.4 Hz, 1H), 3.05 (ddd, J = 24.6, 16.7, 7.5 Hz, 2H), 2.30 (s, 3H), 2.10 (s, 3H), 1.77 (s, 3H). 13C-NMR (151 MHz, CDCl3) δ 205.74, 170.90, 165.74, 155.98, 138.34, 137.28, 132.53, 132.00, 129.85, 129.45, 127.83, 124.10, 117.17, 51.35, 45.64, 30.17, 23.36, 21.08. GC-MS: m/z found: 456 (M calculated for C22H21BrN2O2S: 456); m/z (%) = 459 (M + 1, 5, 81Br), 458 (M, 6, 81Br), 457 (M + 1, 5, 79Br), 456 (M, 5, 79Br), 415 (25, 81Br), 413 (26, 79Br), 373 (26, 81Br), 371 (25, 79Br), 359 (9, 81Br), 357 (11, 79Br), 310 (6, 81Br), 308 (6, 79Br), 268 (13, 81Br), 266 (17, 79Br), 191 (10), 149 (45), 107 (48), 86 (68), 84 (99), 43 (100), 35 (34).
N-Acetyl-4-(furan-2-yl)-4-(phenylamino)butan-2-one (5i): yellow solid, mp: 80 °C, yield: 75%, 1H-NMR (600 MHz, CDCl3) δ 7.32–7.26 (m, 5H), 6.48 (t, J = 7.6 Hz, 1H) overlapped with 6.8–6.3 (m, 1H), 6.25–6.14 (m, 1H), 6.02 (s, 1H), 2.85 (ddd, J = 24.3, 16.0, 7.6 Hz, 1H), 2.15 (s, 3H), 1.73 (s, 3H). 13C-NMR (151 MHz, CDCl3) δ 205.31, 170.46, 152.24, 141.88, 139.27, 129.52, 129.30, 128.50, 110.40, 108.51, 48.48, 44.51, 29.85, 23.22. GC-MS: m/z found: 271 (M calculated for C16H17NO3: 271); m/z (%) = 271 (M, 1); 228 (3); 186 (3); 170 (3); 137 (2); 104 (1); 93 (6); 77 (8); 65 (12); 43 (100); 39 (32).
N-Acetyl-4-(phenylamino)-4-(thiophen-2-yl)butan-2-one (5j): white solid, mp: 84 °C, yield: 74%, 1H-NMR (600 MHz, CDCl3) δ 7.41–7.20 (m, 4H), 7.19 (d, J = 5.1 Hz, 1H), 7.06–6.65 (m, 1H), 6.85 (dd, J = 4.9, 3.6 Hz, 1H), 6.75 (d, J = 3.4 Hz, 1H), 6.56 (dd, J = 8.5, 6.6 Hz, 1H), 2.99 (ddd, J = 22.7, 16.3, 7.6 Hz, 2H), 2.17 (s, 3H), 1.74 (s, 3H). 13C-NMR (151 MHz, CDCl3) δ 205.36, 170.46, 142.75, 139.51, 129.78, 129.38, 128.63, 126.41, 126.23, 125.43, 50.29, 47.11, 30.05, 23.50. GC-MS: m/z found: 287 (M calculated for C16H17NO2S: 287); m/z (%) = 287 (M, 4); 244 (16); 202 (10); 188 (5); 153 (7); 135 (11); 118 (4); 109 (6); 93 (29); 84 (19); 77 (13); 66 (22); 43 (100); 39 (5); 27 (1).
N-Acetyl-4-(phenylamino)-4-(pyridin-3-yl)butan-2-one (5k): white solid, mp: 130 °C, yield: 75%, 1H-NMR (600 MHz, CDCl3) δ 8.71 (d, J = 2.0 Hz, 1H), 8.50 (dd, J = 4.7, 1.4 Hz, 1H), 7.72 (dd, J = 6.3, 1.6 Hz, 1H), 7.24 (dd, J = 7.8, 4.8 Hz, 1H), 7.12 (t, J = 7.9 Hz, 2H), 6.68 (t, J = 7.3 Hz, 1H), 6.53 (d, J = 7.8 Hz, 2H), 6.46 (t, J = 7.6 Hz, 1H), 2.92 (ddd, J = 22.4, 16.3, 7.6 Hz, 2H), 2.12 (s, 3H), 1.74 (s, 3H). 13C-NMR (151 MHz, CDCl3) δ 206.04, 170.2, 148.06, 139.82, 146.4, 137.8, 134.02, 128.93, 123.88, 118.17, 113.78, 52.08, 50.33, 30.93, 23.48. GC-MS: m/z found: 282 (M calculated for C17H18N2O2: 282); m/z (%) = 282 (M, 2); 239 (8); 183 (12); 149 (4); 132 (8); 106 (3); 93 (4); 77 (6); 43 (100); 39 (14); 27 (4).