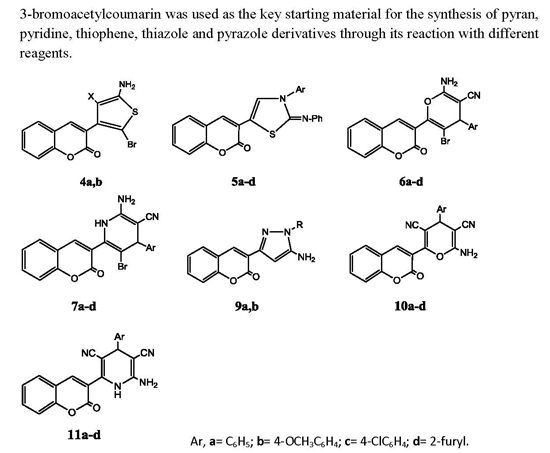
3.1. Chemistry
All melting points were determined on a Stuart apparatus and the values given are uncorrected. IR spectra (KBr, cm
−1) were determined on a Shimadzu IR 435 spectrophotometer (Faculty of Pharmacy, Cairo University, Egypt).
1H-NMR and
13C-NMR spectra were recorded on Bruker Ascend 400 MHz spectrophotometers (Microanalytical Unit, Faculty of Pharmacy, Cairo University, Egypt) using TMS as the internal standard. Chemical shift values were recorded in ppm on δ scale. The electron impact (EI) mass spectra were recorded on a Hewlett Packard 5988 spectrometer (Microanalysis Center, Cairo University, Egypt). Elemental analyses were carried out at the Microanalysis Center, Cairo University, Egypt; found values were within ±0.35% of the theoretical ones. The progress of the reactions was monitored using thin layer chromatography (TLC) sheets precoated with UV fluorescent silica gel Merck 60F 254 and were visualized using UV lamp. The 3-(2-bromoacetyl)-2
H-chromen-2-one (
1) [
30,
31] was obtained using the reported procedure by the reaction of 3-acetylcoumarin in chloroform solution with bromine together with continuous stirring.
3.1.1. Synthesis of 2-oxo-2-(2-oxo-2H-chromen-3-yl)-N'-phenylacetohydrazonoylbromide (2)
To a cold solution of the 3-(2-bromoacetyl)-2H-chromen-2-one (1) (2.67 g, 0.01 mol) in ethanol (30 mL) containing sodium acetate (2.5 g), a cold solution of benzenediazonium chloride (0.01 mol) (prepared by the addition of sodium nitrite solution (0.7 g, 0.01 mol) to a cold solution of aniline (0.93 g, 0.01 mol) in concentrated hydrochloric acid (12 mL) with continuous stirring) was added while stirring. The reaction mixture was kept at room temperature for 1 h and the formed solid product was collected by filtration and crystallized from ethanol. Yield: 85%;m.p.: 88–90 °C; IR (KBr, cm−1): 3425 (NH), 3058 (CH, aromatic), 1726, 1695 (2C=O), 1601 (C=N); 1H-NMR (DMSO-d6): δ 6.81 (s, 1H, coumarin H-4), 6.94–8.13 (m, 9H, C6H5, C6H4), 10.41 (s, 1H, NH, D2O exchangeable); 13C-NMR (DMSO-d6): δ 116.0, 118.9, 119.0, 119.2, 122.6, 124.8, 126.7, 129.6, 132.3, 134.6, 142.0, 143.1 (coumarin, C6H5 C), 164.0, 164.2 (2C=O), 175.3 (C=N); MS: m/z (%) 371 (M+). Anal. Calcd. for C17H11BrN2O3: C, 55.01; H, 2.99; N, 7.55. Found: C, 55.32; H, 3.29; N, 7.33.
3.1.2. Synthesis of 2-(2-Hydroxy-1-(2-oxo-2H-chromen-3-yl)ethylidene)malononitrile (3)
A mixture of 1 (2.67 g, 0.01 mol), malononitrile (0.66 g, 0.1 mol) and ammonium acetate (0.5 g)were heated in an oil bath at 120 °C for 1 h then left to cool. The reaction product was dissolved in ethanol, poured onto ice water and neutralized by hydrochloric acid. The solid product was precipitated, filtered, washed with water, and crystallized from ethanol. Yield: 75%; m.p.: 162–164 °C; IR (KBr, cm−1): 3432 (OH), 3089 (CH, aromatic), 2206 (CN), 1709 (C=O); 1H-NMR (DMSO-d6): δ 5.15 (s, 2H, CH2), 6.95 (s, 1H, coumarin H-4), 7.15–7.96 (m, 4H, C6H4), 10.58 (s, 1H, OH, D2O exchangeable); 13C-NMR (DMSO-d6): δ 61.1 (CH2), 98.6, 102.3 (C=C), 116.8, 117.4 (2CN), 121.3, 123.6, 124.2, 125.8, 126.8, 129.4, 130.2, 132.9 (coumarin C), 163.5 (CO); MS: m/z (%) 252 (M+). Anal. Calcd. for C14H8N2O3: C, 66.67; H, 3.20; N, 11.11. Found: C, 66.32; H, 3.09; N, 11.05.
3.1.3. General Procedure for the Synthesis of 4a,b
A mixture of 1 (2.67 g, 0.01 mol) in absolute ethanol (40 mL) containing triethylamine (1.0 mL) and elemental sulfur (0.32 g, 0.01 mol) and either malononitrile (0.66 g, 0.01 mol) or ethyl cyanoacetate (1.13 g, 0.01 mol) was heated under reflux for 2 h. The reaction mixture was left to cool to room temperature and the formed solid product was collected by filtration and crystallized from ethanol.
2-Amino-5-bromo-4-(2-oxo-2H-chromen-3-yl)thiophene-3-carbonitrile (4a). Yield: 71%; m.p.: 180–182 °C; IR (KBr, cm−1): 3427 (NH2), 3034 (CH, aromatic), 2209 (CN), 1703 (C=O); 1H-NMR (DMSO-d6): δ 3.60 (s, 2H, NH2, D2O exchangeable), 6.90 (s, 1H, coumarin H-4), 7.07–7.85 (m, 4H, C6H4); 13C-NMR (DMSO-d6): δ 116.3 (CN), 119.3, 122.5, 124.2, 126.8, 129.6, 130.2, 134.5, 138.0, 139.8, 140.2, 143.8, 154.2 (coumarin, thiophene C), 166.2 (CO); MS: m/z (%) 347 (M+). Anal. Calcd. for C14H7BrN2O2S: C, 48.43; H, 2.03; N, 8.07; S, 9.24. Found: C, 48.68; H, 2.29; N, 8.39; S, 9.03.
Ethyl 2-amino-5-bromo-4-(2-oxo-2H-chromen-3-yl)thiophene-3-carboxylate (4b). Yield: 61%; m.p.: 177–179 °C; IR (KBr, cm−1): 3438 (NH2), 3089 (CH, aromatic), 1720, 1705 (2C=O); 1H-NMR (DMSO-d6): δ 1.15 (t, 3H, J = 7.2 Hz, CH2–CH3), 3.11 (q, 2H, J = 7.2 Hz, CH2–CH3), 3.69 (s, 2H, NH2, D2O exchangeable), 6.95 (s, 1H, coumarin H-4), 7.35–7.51 (m, 4H, C6H4); 13C-NMR (DMSO-d6): δ 22.3 (ester CH3), 58.7 (ester CH2), 119.3, 121.3, 122.8, 123.5, 124.8, 126.9, 127.3, 129.5, 130.8, 132.5, 134.9, 144.2 (coumarin, thiophene C), 166.0, 166.4 (2CO); MS: m/z (%) 394 (M+). Anal. Calcd. for C16H12BrNO4S: C, 48.74; H, 3.07; N, 3.55; S, 8.13. Found: C, 48.88; H, 3.39; N, 3.88; S, 7.89.
3.1.4. General Procedure for the Synthesis of 5a–d
A mixture of 1 (2.67 g, 0.01 mol), phenylisothiocyante (0.01 mol) and either of aniline (0.35 g, 0.01 mol), p-toluidine (0.04 g, 0.01 mol), 4-methoxyaniline (0.46 g, 0.01 mol) or 4-chloroaniline (0.47 g, 0.01 mol) in absolute ethanol (40 mL) containing triethylamine (1.0 mL) was heated under reflux for 2 h, left to cool to room temperature, poured onto ice/water, and neutralized by hydrochloric acid. The precipitated solid was collected by filtration, washed with water and crystallized from ethanol.
3-(3-Phenyl-2-(phenylimino)-2,3-dihydrothiazol-5-yl)-2H-chromen-2-one (5a). Yield: 76%; m.p.: 158–160 °C; IR (KBr, cm−1): 3064 (CH, aromatic), 1723 (C=O), 1609 (C=N); 1H-NMR (DMSO-d6): δ 3.99 (s, 1H, thiazole H-4), 6.67 (s, 1H, coumarin H-4), 7.43–8.58 (m, 14H, 2C6H5, C6H4); 13C-NMR (DMSO-d6): δ 119.3, 120.8, 121.3, 122.6, 124.3, 124.8, 126.2, 127.0, 127.3, 128.1, 129.2, 130.2, 132.8, 133.2, 138.4, 140.3, 142.8, 144.5 (coumarin, thiazole, 2C6H5 C), 164.3 (CO), 173.4 (C=N); MS: m/z (%) 396 (M+). Anal. Calcd. for C24H16N2O2S: C, 72.71; H, 4.07; N, 7.07; S, 8.09. Found: C, 72.43; H, 4.09; N, 7.29; S, 8.39.
3-(2-(Phenylimino)-3-(p-tolyl)-2,3-dihydrothiazol-5-yl)-2H-chromen-2-one (5b). Yield: 69%; m.p.: 99–101 °C; IR (KBr, cm−1): 3033 (CH, aromatic), 1721 (C=O), 1600 (C=N); 1H-NMR (DMSO-d6): δ 2.25 (s, 3H, CH3), 3.98 (s, 1H, thiazole H-4), 6.60 (s, 1H, coumarin H-4), 7.09–7.50 (m, 13H, C6H5, 2C6H4); 13C-NMR (DMSO-d6): δ 20.8 (CH3), 120.2, 121.4, 121.8, 122.4, 123.9, 124.4, 125.2, 126.9, 128.0, 130.2, 132.5, 133.2, 136.3, 138.8, 141.6, 142.9, 143.4, 144.6 (coumarin, thiazole, C6H5, C6H4 C), 164.1 (CO), 173.8 (C=N); MS: m/z (%) 410 (M+). Anal. Calcd. for C25H18N2O2S: C, 73.15; H, 4.42; N, 6.82; S, 7.81. Found: C, 73.45; H, 4.09; N, 6.69; S, 7.64.
3-(3-(4-Methoxyphenyl)-2-(phenylimino)-2,3-dihydrothiazol-5-yl)-2H-chromen-2-one (5c). Yield: 72%; m.p.: 103–105 °C; IR (KBr, cm−1): 3053 (CH, aromatic), 1717 (C=O), 1603 (C=N); 1H-NMR (DMSO-d6): δ 3.79 (s, 3H, OCH3), 4.45 (s, 1H, thiazole H-4), 6.82 (s, 1H, coumarin H-4), 6.88–7.50 (m, 13H, C6H5, 2C6H4); 13C-NMR (DMSO-d6): δ 32.9 (OCH3), 120.4, 120.9, 121.3, 123.0, 123.6, 124.1, 125.3, 127.3, 128.6, 130.6, 132.8, 136.4, 138.4, 138.9, 139.5, 140.8, 143.6, 144.8 (coumarin, thiazole, C6H5, C6H4C), 164.6 (CO), 173.2 (C=N); MS: m/z (%) 426 (M+). Anal. Calcd. for C25H18N2O3S: C, 70.40; H, 4.25; N, 6.57; S, 7.52. Found: C, 70.13; H, 4.08; N, 6.82; S, 7.29.
3-(3-(4-Chlorophenyl)-2-(phenylimino)-2,3-dihydrothiazol-5-yl)-2H-chromen-2-one (5d). Yield: 71%; m.p.: 123–125 °C; IR (KBr; cm−1): 3030 (CH; aromatic); 1719 (C=O); 1597 (C=N); 1H-NMR (DMSO-d6): δ 3.98 (s, 1H, thiazole H-4); 6.56 (s, 1H, coumarin H-4); 6.99–7.54 (m; 13H, C6H5, 2 C6H4); 13C-NMR (DMSO-d6): δ 119.8, 120.4, 121.2, 122.4, 123.9, 124.6, 125.4, 126.0, 127.6, 128.2, 129.1, 130.3, 131.2, 132.8, 137.3, 140.5, 142.8, 144.4 (coumarin, thiazole, C6H5, C6H4 C); 164.9 (CO), 173.5 (C=N); MS: m/z (%) 430 (M+). Anal. Calcd. for C24H15ClN2O2S: C; 66.90; H; 3.51; N; 6.50; S; 7.44. Found: C; 66.66; H; 3.77; N; 6.82; S; 7.69.
3.1.5. General Procedure for the Synthesis of Compounds 6a–d
A mixture of compound 1 (2.67 g, 0.01 mol), malononitrile (0.66 g, 0.01 mol) and either benzaldehyde (1.06 g, 0.01 mol), 4-methoxybenzaldehyde (1.36 g, 0.01 mol), 4-chlorobenzaldehyde (1.27 g, 0.01 mol) or furfural (0.96 g, 0.01 mol) in absolute ethanol (40 mL) containing triethylamine (1.0 mL) was heated under reflux for 2 h, left to cool to room temperature, poured onto ice/water, and neutralized by hydrochloric acid. The precipitated solid was collected by filtration, washed with water and crystallized from ethanol.
2-Amino-5-bromo-6-(2-oxo-2H-chromen-3-yl)-4-phenyl-4H-pyran-3-carbonitrile (6a). Yield: 68%; m.p.: 140–142 °C; IR (KBr, cm−1): 3408 (NH2), 3063 (CH, aromatic), 2212 (CN), 1723 (C=O); 1H-NMR (DMSO-d6): δ 3.46 (s, 2H, NH2, D2O exchangeable), 5.01 (s, 1H, pyran H-4), 7.02 (s, 1H, coumarin H-4), 7.24–7.98 (m, 9H, C6H5, C6H4); 13C-NMR (DMSO-d6): δ 65.8 (pyran C-4), 116.8 (CN), 121.3, 121.8, 122.4, 122.8, 123.2, 124.7, 126.7, 127.8, 128.3, 129.6, 130.6, 131.8, 133.9, 140.8, 142.3, 143.9 (coumarin, pyran, C6H5 C), 164.9 (CO); MS: m/z (%) 421 (M+). Anal. Calcd. for C21H13BrN2O3: C, 59.88; H, 3.11; N, 6.65. Found: C, 59.58; H, 3.02; N, 6.39.
2-Amino-5-bromo-4-(4-methoxyphenyl)-6-(2-oxo-2H-chromen-3-yl)-4H-pyran-3-carbonitrile (6b). Yield: 65%; m.p.: 193–195 °C; IR (KBr, cm−1): 3415 (NH2), 3070 (CH, aromatic), 2219 (CN), 1720 (C=O); 1H-NMR (DMSO-d6): δ 3.11 (s, 3H, OCH3), 3.46 (s, 2H, NH2, D2O exchangeable), 5.68 (s, 1H, pyran H-4), 7.09 (s, 1H, coumarin H-4), 7.34–7.87 (m, 8H, 2C6H4); 13C-NMR (DMSO-d6): δ 34.8 (OCH3), 65.4 (pyran C-4), 116.8 (CN), 119.3, 121.3, 122.4, 122.8, 123.2, 124.7, 125.1, 125.8, 126.7, 127.8, 129.3, 130.6, 133.9, 140.8, 142.3, 143.9 (coumarin, pyran, C6H4 C), 164.9 (CO); MS: m/z (%) 451 (M+). Anal. Calcd. for C22H15BrN2O4: C, 58.55; H, 3.35; N, 6.21. Found: C, 58.66; H, 3.12; N, 5.91.
2-Amino-5-bromo-4-(4-chlorophenyl)-6-(2-oxo-2H-chromen-3-yl)-4H-pyran-3-carbonitrile (6c). Yield: 65%; m.p.: 178–180 °C; IR (KBr, cm−1): 3410 (NH2), 3067 (CH, aromatic), 2211 (CN), 1720 (C=O); 1H-NMR (DMSO-d6): δ 3.31 (s, 2H, NH2, D2O exchangeable), 5.73 (s, 1H, pyran H-4), 6.73 (s, 1H, coumarin H-4), 6.93–7.72 (m, 8H, 2C6H4); 13C-NMR (DMSO-d6): δ 65.8 (pyran C-4), 116.6 (CN), 119.3, 120.8, 121.6, 122.3, 123.6, 123.9, 125.3, 125.9, 126.8, 127.3, 130.9, 132.2, 138.9, 140.2, 142.6, 143.1 (coumarin, pyran, C6H4 C), 164.6 (CO); MS: m/z (%) 455 (M+). Anal. Calcd. for C21H12BrClN2O3: C, 55.35; H, 2.65; N, 6.15. Found: C, 55.21; H, 2.95; N, 5.93.
2-Amino-5-bromo-4-(furan-2-yl)-6-(2-oxo-2H-chromen-3-yl)-4H-pyran-3-carbonitrile (6d). Yield: 73%; m.p.: 148–150 °C; IR (KBr, cm−1): 3420 (NH2), 3048 (CH, aromatic), 2216 (CN), 1727 (C=O); 1H-NMR (DMSO-d6): δ 3.31 (s, 2H, NH2, D2O exchangeable), 5.80 (s, 1H, pyran H-4), 7.12 (s, 1H, coumarin H-4), 7.30–8.00 (m, 7H, C6H4, furan); 13C-NMR (DMSO-d6): δ 65.8 (pyran C-4), 116.4 (CN), 118.9, 121.8, 122.1, 122.7, 123.2, 124.2, 125.2, 126.0, 126.4, 128.4, 130.9, 134.5, 141.6, 140.6, 143.9, 148.2 (coumarin, pyran, furan C), 164.6 (CO); MS: m/z (%) 411 (M+). Anal. Calcd. for C19H11BrN2O4: C, 55.50; H, 2.70; N, 6.81. Found: C, 55.31; H, 3.01; N, 6.62.
3.1.6. General Procedure for the Synthesis of 7a–d
A mixture of compound 1 (2.67 g, 0.01 mol), malonanitrile (0.66 g, 0.01 mol) and either benzaldehyde (1.06 g, 0.01 mol), 4-methoxybenzaldehyde (1.36 g, 0.01 mol), 4-chlorobenzaldehyde (1.27 g, 0.01 mol) or furfural (0.96 g, 0.01 mol) in absolute ethanol (40 mL) containing ammonium acetate (0.5 g) was heated under reflux for 3 h, left to cool to room temperature, poured onto ice/water, and neutralized with hydrochloric acid. The precipitated solid was collected by filtration, washed with water and crystallized from ethanol.
2-Amino-5-bromo-6-(2-oxo-2H-chromen-3-yl)-4-phenyl-1,4-dihydropyridine-3-carbonitrile (7a). Yield: 80%; m.p.: 171–173 °C; IR (KBr, cm−1): 3415–3346 (NH2, NH), 3064 (CH, aromatic), 2209 (CN), 1714 (C=O); 1H-NMR (DMSO-d6): δ 3.48 (s, 2H, NH2, D2O exchangeable), 7.15 (s, 1H, pyridine H-4), 7.20 (s, 1H, coumarin H-4), 7.34–7.69 (m, 9H, C6H5, C6H4), 9.16 (s, 1H, NH, D2O exchangeable); 13C-NMR (DMSO-d6): δ 62.3 (pyridine C-4), 116.9 (CN), 119.3, 120.3, 121.9, 123.8, 124.5, 124.8, 125.8, 126.2, 126.9, 128.0, 128.3, 130.3, 132.4, 139.3, 140.9, 143.2 (coumarin, pyridine, C6H5 C), 165.3 (CO); MS: m/z (%) 420 (M+). Anal. Calcd. for C21H14BrN3O2: C, 60.02; H, 3.36; N, 10.00. Found: C, 59.89; H, 3.18; N, 9.73.
2-Amino-5-bromo-4-(4-methoxyphenyl)-6-(2-oxo-2H-chromen-3-yl)-1,4-dihydropyridine-3-carbonitrile (7b). Yield: 82%; m.p.: 164–166 °C; IR (KBr, cm−1): 3407–3365 (NH2, NH), 3064 (CH, aromatic), 2207 (CN), 1710 (C=O); 1H-NMR (DMSO-d6): δ 3.66 (s, 3H, OCH3), 3.84 (s, 2H, NH2, D2O exchangeable), 6.92 (s, 1H, pyridine H-4), 6.95 (s, 1H, coumarin H-4), 6.97–7.98 (m, 8H, 2C6H4), 9.86 (s, 1H, NH, D2O exchangeable); 13C-NMR (DMSO-d6): δ 38.9 (OCH3), 62.8 (pyridine C-4), 116.3 (CN), 120.1, 120.3, 122.6, 123.2, 124.5, 124.6, 125.8, 126.8, 127.4, 129.8, 132.6, 136.2, 137.4, 139.2, 140.6, 143.8 (coumarin, pyridine, C6H4 C), 164.4 (CO); MS: m/z (%) 450 (M+). Anal. Calcd. for C22H16BrN3O3: C, 58.68; H, 3.58; N, 9.33. Found: C, 58.38; H, 3.28; N, 9.67.
2-Amino-5-bromo-4-(4-chlorophenyl)-6-(2-oxo-2H-chromen-3-yl)-1,4-dihydropyridine-3-carbonitrile (7c). Yield: 81%; m.p.: 206–208 °C;IR (KBr, cm−1): 3412–3360 (NH2, NH), 3055 (CH, aromatic), 2183 (CN), 1704 (C=O); 1H-NMR (DMSO-d6): δ 3.88 (s, 2H, NH2, D2O exchangeable), 6.92 (s, 1H, pyridine H-4), 6.95 (s, 1H, coumarin H-4), 6.98–7.94 (m, 8H, 2C6H4), 10.00 (s, 1H, NH, D2O exchangeable); 13C-NMR (DMSO-d6): δ 62.8 (pyridine C-4), 116.8 (CN), 120.4, 121.8, 122.9, 123.1, 124.3, 125.4, 126.9, 127.5, 128.3, 130.5, 133.2, 135.4, 136.8, 139.7, 140.2, 142.5 (coumarin, pyridine, C6H4 C), 164.8 (CO); MS: m/z (%) 454 (M+). Anal. Calcd. for C21H13BrClN3O2: C, 55.47; H, 2.88; N, 9.24. Found: C, 55.19; H, 3.08; N, 9.05.
2-Amino-5-bromo-4-(furan-2-yl)-6-(2-oxo-2H-chromen-3-yl)-1,4-dihydropyridine-3-carbonitrile (7d). Yield: 83%; m.p.: 205–207 °C; IR (KBr, cm−1): 3425–3387 (NH2, NH), 3045 (CH, aromatic), 2210 (CN), 1709 (C=O); 1H-NMR (DMSO-d6): δ 3.92 (s, 2H, NH2, D2O exchangeable), 6.46 (s, 1H, pyridine H-4), 6.77 (s, 1H, coumarin H-4), 6.90–8.08 (m, 7H, C6H4, furan), 8.82 (s, 1H, NH, D2O exchangeable); 13C-NMR (DMSO-d6): δ 63.0 (pryidine C-4), 116.9 (CN), 120.8, 121.3, 122.7, 123.5, 124.8, 125.6, 126.6, 127.2, 128.1, 129.2, 129.6, 130.6, 133.3, 135.3, 138.9, 144.7 (coumarin, pyridine, furan C), 166.2 (CO); MS: m/z (%) 410 (M+). Anal. Calcd. for C19H12BrN3O3: C, 55.63; H, 2.95; N, 10.24. Found: C, 55.39; H, 3.11; N, 10.51.
3.1.7. Synthesis of 3-oxo-3-(2-oxo-2H-chromen-3-yl)propanenitrile (8)
A solution of compound 1 (2.67 g, 0.01 mol) in absolute ethanol (40 mL) was heated at 60 °C, then added to a solution of KCN (0.65 g, 0.01 mol in 10 mL water). The mixture was stirred for 0.5 h and the product was precipitated by adding ice and few drops of hydrochloric acid. The precipitated solid was collected by filtration, washed with water and crystallized from ethanol. Yield: 85%; m.p.: 158–160 °C; IR (KBr, cm−1): 3091 (CH, aromatic), 2247 (CN), 1739 (C=O); 1H-NMR (DMSO-d6): δ 5.08 (s, 2H, CH2), 6.63 (s, 1H, coumarin H-4), 6.88–7.83 (m, 4H, C6H4); 13C-NMR (DMSO-d6): δ 61.1 (CH2), 116.3 (CN), 121.0, 122.6, 123.8, 125.0, 126.2, 127.2, 129.4, 130.3, 133.2 (coumarin C), 162.2 (CO). MS: m/z (%) 213 (M+). Anal. Calcd. for C12H7NO3: C, 67.61; H, 3.31; N, 6.57. Found: C, 67.35; H, 3.11; N, 6.78.
3.1.8. General Procedure for the Synthesis of Compounds 9a,b
A solution of compound 8 (2.13 g, 0.01 mol) and either hydrazine hydrate (0.5 g, 0.01 mol) or phenylhydrazine (1.08 g, 0.01 mol) in absolute ethanol (40 mL) was heated under reflux for 2 h, left to cool to room temperature, poured onto ice/water containing few drops hydrochloric acid. The resulting product was collected by filtration, washed with water and crystallized from ethanol.
3-(5-Amino-1H-pyrazol-3-yl)-2H-chromen-2-one (9a). Yield: 83%;m.p.: 218–220 °C; IR (KBr, cm−1): 3416–3368 (NH2, NH), 3044(CH, aromatic), 1718 (C=O), 1611 (C=N); 1H-NMR (DMSO-d6): δ 3.92 (s, 2H, NH2, D2O exchangeable), 6.81 (s, 1H, pyrazole H-4), 6.90 (s, 1H, coumarin H-4), 6.93–7.84 (m, 4H, C6H4), 11.19 (s, 1H, NH, D2O exchangeable); 13C-NMR (DMSO-d6): δ 121.0, 122.6, 124.2, 125.9, 129.0, 130.6, 133.3, 135.3, 138.9, 140.2 (coumarin, pyrazole C), 165.3 (CO), 172.6 (C=N); MS: m/z (%) 227 (M+). Anal. Calcd. for C12H9N3O2: C, 63.43; H, 3.99; N, 18.49. Found: C, 63.52; H, 4.25; N, 18.22.
3-(5-Amino-1-phenyl-1H-pyrazol-3-yl)-2H-chromen-2-one (9b) Yield: 85%; m.p.: 158–160 °C; IR (KBr, cm−1): 3430 (NH2), 3056 (CH, aromatic), 1721 (C=O), 1607 (C=N); 1H-NMR (DMSO-d6): δ 3.88 (s, 2H, NH2, D2O exchangeable), 6.85 (s, 1H, pyrazole H-4), 6.89 (s, 1H, coumarin H-4), 6.95–7.83 (m, 9H, C6H5, C6H4); 13C-NMR (DMSO-d6): δ 120.3, 121.3, 122.9, 123.5, 124.8, 126.4, 127.4, 130.8, 131.4, 133.2, 135.6, 133.1, 136.5, 138.0 (coumarin, pyrazole, C6H5 C), 165.8 (CO), 172.3 (C=N); MS: m/z (%) 303 (M+). Anal. Calcd. for C18H13N3O2: C, 71.28; H, 4.32; N, 13.85. Found: C, 71.53; H, 4.09; N, 13.92.
3.1.9. General Procedure for the Synthesis of Compounds 10a–d
A mixture of compound 8 (2.67 g, 0.01 mol), malonanitrile (0.66 g, 0.01 mol) and either benzaldehyde (1.06 g, 0.01 mol), 4-methoxybenzaldehyde (1.36 g, 0.01 mol), 4-chlorobenzaldehyde (1.27 g, 0.01 mol) or furfural (0.96 g, 0.01 mol) in absolute ethanol (40 mL) containing triethylamine (1.0 mL) was heated under reflux for 2 h, left to cool to room temperature, poured onto ice/water, and neutralized by hydrochloric acid. The precipitated solid was collected by filtration, washed with water and crystallized from ethanol.
2-Amino-6-(2-oxo-2H-chromen-3-yl)-4-phenyl-4H-pyran-3,5-dicarbonitrile (10a). Yield: 88%; m.p.: 173–175 °C; IR (KBr, cm−1): 3432 (NH2), 3064 (CH, aromatic), 2200 (CN), 1723 (C=O); 1H-NMR (DMSO-d6): δ 3.73 (s, 2H, NH2, D2O exchangeable), 6.78 (s, 1H, pyran H-4), 6.85 (s, 1H, coumarin H-4), 7.14–7.92 (m, 9H, C6H5, C6H4); 13C-NMR (DMSO-d6): δ 62.8 (pyran C-4), 116.3, 117.3 (2CN), 119.8, 120.8, 123.2, 124.2, 125.1, 126.8, 127.9, 128.4, 129.3, 130.1, 132.3, 133.4, 134.8, 135.1, 138.2, 140.6 (coumarin, pyran, C6H5 C), 164.2 (CO); MS: m/z (%) 367 (M+). Anal. Calcd. for C22H13N3O3: C, 71.93; H, 3.57; N, 11.44. Found: C, 71.65; H, 3.88; N, 11.42.
2-Amino-4-(4-methoxyphenyl)-6-(2-oxo-2H-chromen-3-yl)-4H-pyran-3,5-dicarbonitrile (10b). Yield: 75%; m.p.: 113–115 °C; IR (KBr, cm−1): 3431 (NH2), 3053 (CH, aromatic), 2217 (CN), 1726 (C=O); 1H-NMR (DMSO-d6): δ 3.74 (s, 2H, NH2, D2O exchangeable), 3.88 (s, 3H, OCH3), 6.75 (s, 1H, pyran H-4), 6.89 (s, 1H, coumarin H-4), 6.95–7.99 (m, 8H, 2C6H4); 13C-NMR (DMSO-d6): δ 28.9 (OCH3), 63.1 (pyran C-4), 115.9, 116.2 (2CN), 118.3, 119.6, 121.8, 122.9, 123.5, 124.0, 125.3, 126.5, 127.2, 129.5, 130.4, 132.4, 134.2, 135.2, 136.8, 141.2 (coumarin, pyran, C6H4 C), 163.8 (CO). MS: m/z (%) 397 (M+). Anal. Calcd. for C23H15N3O4: C, 69.52; H, 3.80; N, 10.57. Found: C, 69.38; H, 3.62; N, 10.29.
2-Amino-4-(4-chlorophenyl)-6-(2-oxo-2H-chromen-3-yl)-4H-pyran-3,5-dicarbonitrile (10c). Yield: 88%; m.p.: 178–180 °C; IR (KBr, cm−1): 3432 (NH2), 3046 (CH, aromatic), 2198 (CN), 1725 (C=O); 1H-NMR (DMSO-d6): δ 3.89 (s, 2H, NH2, D2O exchangeable), 6.77 (s, 1H, pyran H-4), 6.89 (s, 1H, coumarin H-4), 6.98–8.05 (m, 8H, 2C6H4); 13C-NMR (DMSO-d6): δ 63.7 (pyran C-4), 116.2, 116.8 (2CN), 119.1, 119.6, 122.4, 123.1, 123.8, 124.7, 125.9, 127.3, 129.3, 131.1, 133.6, 134.8, 137.2, 138.2, 138.6, 141.8 (coumarin, pyran, C6H4 C), 164.9 (CO); MS: m/z (%) 401 (M+). Anal. Calcd. for C22H12ClN3O3: C, 65.76; H, 3.01; N, 10.46. Found: C, 65.42; H, 3.29; N, 10.72.
2-Amino-4-(furan-2-yl)-6-(2-oxo-2H-chromen-3-yl)-4H-pyran-3,5-dicarbonitrile (10d). Yield: 84%; m.p.: 133–135 °C; IR (KBr, cm−1): 3426 (NH2), 3033 (CH, aromatic), 2214 (CN), 1724 (C=O); 1H-NMR (DMSO-d6): δ 3.88 (s, 2H, NH2, D2O exchangeable), 6.51 (s, 1H, pyran H-4), 6.54 (s, 1H, coumarin H-4), 6.77–8.29 (m, 7H, C6H4, furan); 13C-NMR (DMSO-d6): δ 62.9 (pyran C-4), 116.4, 116.9 (2CN), 120.4, 122.4, 123.1, 123.8, 125.3, 127.3, 128.9, 129.4, 134.6, 136.2, 137.8, 138.6, 139.3, 140.1, 141.8, 144.8 (coumarin, pyran, furan C), 164.9 (CO); MS: m/z (%) 357 (M+). Anal. Calcd. for C20H11N3O4: C, 67.23; H, 3.10; N, 11.76. Found: C, 67.55; H, 2.86; N, 11.81.
3.1.10. General Procedure for the Synthesis of Compounds 11a–d
A mixture of compound 8 (2.67 g, 0.01 mol), malonanitrile (0.66 g, 0.01 mol) and either benzaldehyde (1.06 g, 0.01 mol), 4-methoxybenzaldehyde (1.36 g, 0.01 mol), 4-chlorobenzaldehyde (1.27 g, 0.01 mol) or furfural (0.96 g, 0.01 mol) in absolute ethanol (40 mL) containing ammonium acetate (0.5 g) was heated under reflux for 3 h, left to cool to room temperature, poured onto ice/water, and neutralized by hydrochloric acid. The precipitated solid was collected by filtration, washed with water and crystalized from ethanol.
2-Amino-6-(2-oxo-2H-chromen-3-yl)-4-phenyl-1,4-dihydropyridine-3,5-dicarbonitrile (11a) Yield: 85%; m.p.: 133–135 °C; IR (KBr, cm−1): 3430–3378 (NH2, NH), 3079 (CH, aromatic), 2197 (CN), 1716 (C=O); 1H-NMR (DMSO-d6): δ 3.88 (s, 2H, NH2, D2O exchangeable), 6.78 (s, 1H, pyridine H-4), 6.92 (s, 1H, coumarin H-4), 6.95–7.96 (m, 9H, C6H5, C6H4), 8.60 (s, 1H, NH, D2O exchangeable); 13C-NMR (DMSO-d6): δ 64.2 (pyridine C-4), 116.2, 116.8 (2CN), 119.1, 119.6, 120.8, 121.6, 122.4, 123.1, 123.8, 125.9, 127.3, 128.0, 129.2, 130.7, 133.6, 134.8, 137.2, 141.8 (coumarin, pyridine, C6H5 C), 164.3 (CO); (MS: m/z (%) 366 (M+). Anal. Calcd. for C22H14N4O2: C, 72.12; H, 3.85; N, 15.29. Found: C, 72.38; H, 4.13; N, 15.05.
2-Amino-4-(4-methoxyphenyl)-6-(2-oxo-2H-chromen-3-yl)-1,4-dihydropyridine-3,5-dicarbonitrile (11b). Yield: 90%; m.p.: 99–101 °C; IR (KBr, cm−1): 3429–3382 (NH2, NH), 3054 (CH, aromatic), 2221 (CN), 1720 (C=O); 1H-NMR (DMSO-d6): δ 3.75 (s, 3H, OCH3), 3.88 (s, 2H, NH2, D2O exchangeable), 6.77 (s, 1H, pyridine H-4), 6.87 (s, 1H, coumarin H-4), 6.90–7.99 (m, 8H, 2C6H4), 8.39 (s, 1H, NH, D2O exchangeable); 13C-NMR (DMSO-d6): δ 33.8 (OCH3), 63.8 (pyridine C-4), 116.0, 116.6 (2CN), 119.6, 120.8, 122.6, 122.9, 123.2, 124.6, 125.9, 126.2, 128.6, 128.8, 129.8, 130.9, 132.8, 134.3, 137.2, 144.2 (coumarin, pyridine, C6H4 C), 164.9 (CO); MS: m/z (%) 396 (M+). Anal. Calcd. for C23H16N4O3: C, 69.69; H, 4.07; N, 14.13. Found: C, 69.38; H, 4.09; N, 14.39.
2-Amino-4-(4-chlorophenyl)-6-(2-oxo-2H-chromen-3-yl)-1,4-dihydropyridine-3,5-dicarbonitrile (11c). Yield: 85%; m.p.: 197–199 °C; IR (KBr, cm−1): 3443–3375 (NH2, NH), 3054 (CH, aromatic), 2200 (CN), 1709 (C=O); 1H-NMR (DMSO-d6): δ 3.86 (s, 2H, NH2, D2O exchangeable), 6.74 (s, 1H, pyridine H-4), 6.96 (s, 1H, coumarin H-4), 7.09–7.97 (m, 8H, 2 C6H4), 10.00 (s, 1H, NH, D2O exchangeable); 13C-NMR (DMSO-d6): δ 63.9 (pyridine C-4), 116.2, 116.8 (2CN), 119.8, 120.3, 121.4, 122.6, 124.9, 125.2, 127.8, 129.3, 132.4, 133.0, 134.1, 137.2, 138.0, 139.3, 139.9, 144.0 (coumarin, pyridine, C6H4 C), 163.0 (CO); MS: m/z (%) 400 (M+). Anal. Calcd. for C22H13ClN4O2: C, 65.92; H, 3.27; N, 13.98. Found: C, 66.22; H, 3.02; N, 13.83.
2-Amino-4-(furan-2-yl)-6-(2-oxo-2H-chromen-3-yl)-1,4-dihydropyridine-3,5-dicarbonitrile (11d). Yield: 85%; m.p.: 168–170 °C; IR (KBr, cm−1): 3427–3375 (NH2, NH), 3034 (CH, aromatic), 2214 (CN), 1715 (C=O); 1H-NMR (DMSO-d6): δ 3.84 (s, 2H, NH2, D2O exchangeable), 6.55 (s, 1H, pyridine H-4), 6.90 (s, 1H, coumarin H-4), 6.98–8.09 (m, 7H, C6H4, furan), 8.81 (s, 1H, NH, D2O exchangeable); 13C-NMR (DMSO-d6): δ 63.8 (pyridine C-4), 116.3, 116.9 (2CN), 119.2, 120.7, 121.8, 122.3, 123.3, 126.9, 127.3, 128.3, 129.9, 130.6, 131.4, 132.8, 134.3, 136.4, 138.2, 143.4 (coumarin, pyridine, furan C), 164.8 (CO); MS: m/z (%) 356 (M+). Anal. Calcd. for C20H12N4O3: C, 67.41; H, 3.39; N, 15.72. Found: C, 67.66; H, 3.59; N, 15.88.