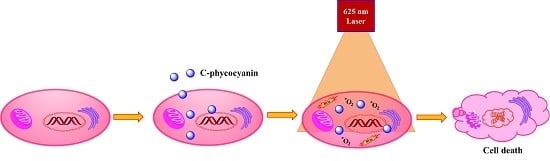
In Vitro Photodynamic Effect of Phycocyanin against Breast Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cellular Morphology
2.2. Differentiation of Cell Death
2.3. Nuclei Cleavage
2.4. Intra-Cellular ROS Generation
2.5. Mitochondrial Membrane Potential
2.6. Apoptosis Assay
3. Methodology
3.1. Materials
3.2. SOSG Assay
3.3. DPBF Assay
3.4. Cellular Uptake
3.5. In Vitro Toxicity
3.6. In Vitro PDT Cell Toxicity
3.7. Morphology of PDT Treated Cells
3.8. Acridine Orange-Ethidium Bromide Staining
3.9. Hoechst Staining
3.10. DCFH-DA Staining
3.11. Rho-123 Staining
3.12. Annexin V-FITC Staining
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bonnett, R.; Martınez, G. Photobleaching of sensitisers used in photodynamic therapy. Tetrahedron 2001, 57, 9513–9547. [Google Scholar] [CrossRef]
- Ormond, A.B.; Freeman, H.S. Dye sensitizers for photodynamic therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef]
- Wainwright, M. Photodynamic therapy—From dyestuffs to high—Tech clinical practice. Rev. Prog. Color. Relat. Top. 2004, 34, 95–109. [Google Scholar] [CrossRef]
- Sharman, W.M.; Allen, C.M.; van Lier, J.E. Role of activated oxygen species in photodynamic therapy. Method Enzymol. 2000, 319, 376–400. [Google Scholar]
- Kruspe, S.; Meyer, C.; Hahn, U. Chlorin e6 conjugated interleukin-6 receptor aptamers selectively kill target cells upon irradiation. Mol. Ther. Nucleic Acids 2014, 3, e143. [Google Scholar] [CrossRef] [PubMed]
- Pleonsil, P.; Soogarun, S.; Suwanwong, Y. Anti-oxidant activity of holo-and apo-c-phycocyanin and their protective effects on human erythrocytes. Int. J. Biol. Macromol. 2013, 60, 393–398. [Google Scholar] [CrossRef] [PubMed]
- El-Baky, H.H.A. Over production of phycocyanin pigment in blue green alga Spirulina sp. and it’s inhibitory effect on growth of ehrlich ascites carcinoma cells. J. Med. Sci 2003, 3, 314–324. [Google Scholar]
- Wu, F.; Zang, X.; Zhang, X.; Zhang, R.; Huang, X.; Hou, L.; Jiang, M.; Liu, C.; Pang, C. Molecular cloning of CPCU and heterodimeric bilin lyase activity analysis of CPCU and CPCS for attachment of phycocyanobilin to Cys-82 on the β-subunit of phycocyanin in arthrospira platensis fachb314. Molecules 2016, 21, 357. [Google Scholar] [CrossRef] [PubMed]
- Muthulakshmi, M.; Saranya, A.; Sudha, M.; Selvakumar, G. Extraction, partial purification, and antibacterial activity of phycocyanin from Spirulina isolated from fresh water body against various human pathogens. J. Algal Biomass Util. 2012, 3, 7–11. [Google Scholar]
- Chen, J.-C.; Liu, K.S.; Yang, T.-J.; Hwang, J.-H.; Chan, Y.-C.; Lee, I.-T. Spirulina and c-phycocyanin reduce cytotoxicity and inflammation-related genes expression of microglial cells. Nutr. Neurosci. 2012, 15, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-W.; Yang, T.-S.; Chen, M.-J.; Chang, Y.-C.; Eugene, I.; Wang, C.; Ho, C.-L.; Lai, Y.-J.; Yu, C.-C.; Chou, J.-C. Purification and immunomodulating activity of c-phycocyanin from Spirulina platensis cultured using power plant flue gas. Process Biochem. 2014, 49, 1337–1344. [Google Scholar] [CrossRef]
- Zheng, S.; Chai, X.; He, L. Faming Zhuanli Shenqing Gongkai Shuomingshu. Chem. Abstr. 1995, 122, 182179h. [Google Scholar]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) in skin: Stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar] [PubMed]
- Vadiraja, B.B.; Gaikwad, N.W.; Madyastha, K. Hepatoprotective effect of c-phycocyanin: Protection for carbon tetrachloride and R-(+)-pulegone-mediated hepatotoxicty in rats. Biochem. Biophys. Res. Commun. 1998, 249, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Dwivedi, U.N.; Khandelwal, S. C-phycocyanin: An effective protective agent against thymic atrophy by tributyltin. Toxicol. Lett. 2011, 204, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Jose, G.P.; Santra, S.; Mandal, S.K.; Sengupta, T.K. Singlet oxygen mediated DNA degradation by copper nanoparticles: Potential towards cytotoxic effect on cancer cells. J. Nanobiotechnol. 2011, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- He, J.-A.; Hu, Y.-Z.; Jiang, L.-J. Photodynamic action of phycobiliproteins: In situ generation of reactive oxygen species. BBA-Bioenergetics 1997, 1320, 165–174. [Google Scholar] [CrossRef]
- Ohyashiki, T.; Nunomura, M.; Katoh, T. Detection of superoxide anion radical in phospholipid liposomal membrane by fluorescence quenching method using 1,3-diphenylisobenzofuran. BBA-Biomembranes 1999, 1421, 131–139. [Google Scholar] [CrossRef]
- Riesenberg, R.; Fuchs, C.; Kriegmair, M. Photodynamic effects of 5-aminolevulinic acid-induced porphyrin on human bladder carcinoma cells in vitro. Eur. J. Cancer 1996, 32, 328–334. [Google Scholar] [CrossRef]
- Pentón-Rol, G.; Marín-Prida, J.; Pardo-Andreu, G.; Martínez-Sánchez, G.; Acosta-Medina, E.F.; Valdivia-Acosta, A.; Lagumersindez-Denis, N.; Rodríguez-Jiménez, E.; Llópiz-Arzuaga, A.; López-Saura, P.A. C-phycocyanin is neuroprotective against global cerebral ischemia/reperfusion injury in gerbils. Brain Res. Bull. 2011, 86, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Zamzami, N.; Kroemer, G. Apoptosis: Condensed matter in cell death. Nature 1999, 401, 127–128. [Google Scholar] [PubMed]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.-K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.P.; Xia, Q.; Hwang, H.-M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Berlett, B.S. Reactive oxygen-mediated protein oxidation in aging and disease. Chem. Res. Toxicol. 1997, 10, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-J.; Kim, E.J.; Kim, S.; Jung, E.M.; Park, J.-W.; Jeong, S.H.; Park, S.E.; Yoo, Y.H.; Kwon, T.K. Caspase-dependent and caspase-independent apoptosis induced by evodiamine in human leukemic U937 cells. Mol. Cancer Ther. 2006, 5, 2398–2407. [Google Scholar] [CrossRef] [PubMed]
- MubarakAli, D.; Gopinath, V.; Rameshbabu, N.; Thajuddin, N. Synthesis and characterization of CdS nanoparticles using c-phycoerythrin from the marine cyanobacteria. Mater. Lett. 2012, 74, 8–11. [Google Scholar] [CrossRef]
- Kong, C.-Z.; Zhang, Z. Bcl-2 overexpression inhibits generation of intracellular reactive oxygen species and blocks adriamycin-induced apoptosis in bladder cancer cells. Asian Pac. J. Cancer Prev. 2013, 14, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Kim, H.P.; Hoetzel, A.; Park, J.W.; Nakahira, K.; Wang, X.; Choi, A.M. Mechanisms of cell death in oxidative stress. Antioxid. Redox Signal. 2007, 9, 49–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, X.; Zeng, Q.; Zhang, Y.; Tu, L.; Liu, T.; Kong, X.; Wang, Y.; Cao, F.; Lambrechts, S.A. Covalently assembled NIR nanoplatform for simultaneous fluorescence imaging and photodynamic therapy of cancer cells. ACS Nano 2012, 6, 4054–4062. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compound is available from the authors.








© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bharathiraja, S.; Seo, H.; Manivasagan, P.; Santha Moorthy, M.; Park, S.; Oh, J. In Vitro Photodynamic Effect of Phycocyanin against Breast Cancer Cells. Molecules 2016, 21, 1470. https://doi.org/10.3390/molecules21111470
Bharathiraja S, Seo H, Manivasagan P, Santha Moorthy M, Park S, Oh J. In Vitro Photodynamic Effect of Phycocyanin against Breast Cancer Cells. Molecules. 2016; 21(11):1470. https://doi.org/10.3390/molecules21111470
Chicago/Turabian StyleBharathiraja, Subramaniyan, Hansu Seo, Panchanathan Manivasagan, Madhappan Santha Moorthy, Suhyun Park, and Jungwan Oh. 2016. "In Vitro Photodynamic Effect of Phycocyanin against Breast Cancer Cells" Molecules 21, no. 11: 1470. https://doi.org/10.3390/molecules21111470