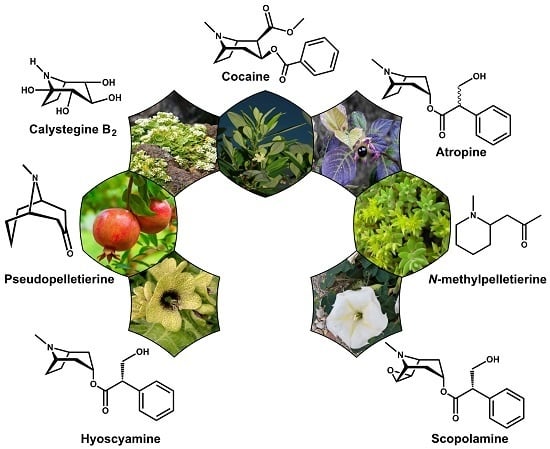
Tropane and Granatane Alkaloid Biosynthesis: A Systematic Analysis
Abstract
:1. Introduction
1.1. Similarities and Differences in Medicinal Properties
1.2. The Scattered Distribution of Tropanes and Granatanes amongst Angiosperms
1.3. Biosynthesis of TAs and GAs
2. Tropane Alkaloid Biosynthesis
3. Granatane Alkaloid Biosynthesis
4. Metabolic Engineering
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pichersky, E.; Lewinsohn, E. Convergent evolution in plant specialized metabolism. Annu. Rev. Plant Biol. 2011, 62, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Nocquet, P.-A.; Opatz, T. Total synthesis of (±)-scopolamine: Challenges of the tropane ring. Eur. J. Org. Chem. 2016, 2016, 1156–1164. [Google Scholar] [CrossRef]
- Dillehay, T.D.; Rossen, J.; Ugent, D.; Karathanasis, A.; Vásquez, V.; Netherly, P.J. Early Holocene coca chewing in northern Peru. Antiquity 2010, 84, 939–953. [Google Scholar] [CrossRef]
- Oberlies, N.H.; Paine, M.F. Antimicrobial activities of Pomegranate. In Pomegranates: Ancient Roots to Modern Medicine; Seeram, N.P., Schulman, R.N., Heber, D., Eds.; American Chemical Society: Boca Raton, FL, USA, 2007. [Google Scholar]
- Jirschitzka, J.; Schmidt, G.W.; Reichelt, M.; Schneider, B.; Gershenzon, J.; D’Auria, J.C. Plant tropane alkaloid biosynthesis evolved independently in the Solanaceae and Erythroxylaceae. Proc. Natl. Acad. Sci. USA 2012, 109, 10304–10309. [Google Scholar] [CrossRef] [PubMed]
- Lounasmaa, M.; Tamminen, T. The tropane alkaloids. In The Alkaloids; Cordell, G.A., Ed.; Academic Press: New York, NY, USA, 1993. [Google Scholar]
- Lazny, R.; Ratkiewicz, A.; Nodzewska, A.; Wynimko, A.; Siergiejczyk, L. Determination of the N-methyl stereochemistry in tropane and granatane derivatives in solution: A computational and NMR spectroscopic study. Tetrahedron 2012, 68, 6158–6163. [Google Scholar] [CrossRef]
- Wink, M. Modes of actions of alkaloids. In Alkaloids; Roberts, M.F., Wink, M., Eds.; Plenum Press: New York, NY, USA, 1998; pp. 301–326. [Google Scholar]
- Schmeller, T.; Sporer, F.; Sauerwein, M.; Wink, M. Binding of tropane alkaloids to nicotinic and muscarinic acetylcholine receptors. Pharmazie 1995, 50, 493–495. [Google Scholar] [PubMed]
- Shakeran, Z.; Keyhanfar, M.; Asghari, G.; Ghanadian, M. Improvement of atropine production by different biotic and abiotic elicitors in hairy root cultures of Datura metel. Turk. J. Biol. 2015, 39, 111–118. [Google Scholar] [CrossRef]
- Xia, K.; Lui, X.; Zhang, Q.; Qiang, W.; Guo, J.; Lan, X.; Chen, M.; Liao, Z. Promoting scopolamine biosynthesis in transgenic Atropa belladonna plants with pmt and h6h overexpression under field conditions. Plant Physiol. Biochem. 2016, 106, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Qiang, W.; Xia, K.; Zhang, Q.; Zeng, J.; Huang, Y.; Yang, C.; Chen, M.; Liu, X.; Lan, X.; Liao, Z. Functional characterisation of a tropine-forming reductase gene from Brugmansia arborea, a woody plant species producing tropane alkaloids. Phytochemistry 2016, 127, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lui, W.; Guo, Z.-X.; Chen, B.-L. Fingerprint analysis of Daturae Flos using rapid resolution liquid chromatography-electrospray ionization mass spectrometry combined with stoichiometry. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 137–142. [Google Scholar] [CrossRef]
- Zeng, S.M.; She, Y.X.; Jiao, B.N.; Liu, G.Y.; Wang, J.; Su, X.S.; Ma, X.B.; Jin, M.J.; Jin, F.; Wang, S.S. Molecularly imprinted polymer for selective extraction and simultaneous determination of four tropane alkaloids from Przewalskia tangutica Maxim. fruit extracts using LC-MS/MS. RSC Adv. 2015, 5, 94997–95006. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Liu, G.; Sun, X.; Zhou, Y.; Deng, X.; Liao, Q.; Xie, Z. Simultaneous determination of atropine, scopolamine, and anisodamine from Hyoscyamus niger L. in rat plasma by high-performance liquid chromatography with tandem mass spectrometry and its application to a pharmacokinetics study. J. Sep. Sci. 2014, 37, 2664–2674. [Google Scholar] [CrossRef] [PubMed]
- Mann, J. Murder, Magic, and Medicine; Oxford University Press: New York, NY, USA, 1992; p. 232. [Google Scholar]
- Wink, M. A short history of alkaloids. In Alkaloids; Roberts, M.F., Wink, M., Eds.; Plenum Press: New York, NY, USA, 1998; pp. 11–44. [Google Scholar]
- Carroll, F.I.; Gao, Y.; Abraham, P.; Lewin, A.H.; Lew, R.; Patel, A.; Boja, J.W.; Kuhar, M.J. Probes for the cocaine receptor. Potentially irreversible ligands for the dopamine transporter. J. Med. Chem. 1992, 35, 1813–1817. [Google Scholar] [CrossRef] [PubMed]
- Carroll, F.I.; Lewin, A.H.; Boja, J.W.; Kuhar, M.J. Cocaine receptor: Biochemical characterization and structure-activity relationships of cocaine analogs at the dopamine transporter. J. Med. Chem. 1992, 35, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Sidorowicz, K.; Lazny, R. Structural studies of cyclic β-amino ketons using computational and NMR methods. CHEMIK 2015, 69, 401–410. [Google Scholar]
- Krunic, A.; Pan, D.; Dunn, W.J., 3rd; Mariappan, S.V. The stereochemistry of N-methyl and aryl substituents determine the biological activities of 3-aryl-8-methyl-8-azabicyclo[3.2.1]oct-2,3-enes. Bioorg. Med. Chem. 2009, 17, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Fozard, J. The peripheral actions of 5-hydroxytryptamine. In The Developement and Early Clinical Evaluation of Selective 5-HT3 Receptor Antagonsts; Oxford University Press: Oxford, UK; New York, NY, USA, 1989; pp. 354–376. [Google Scholar]
- Aapro, M. Granisetron: An update on its clinical use in the management of nausea and vomiting. Oncologist 2004, 9, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Balfour, J.A.; Goa, K.L. Dolasetron. A review of its pharmacology and therapeutic potential in the management of nausea and vomiting induced by chemotherapy, radiotherapy or surgery. Drugs 1997, 54, 273–298. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.H.; Pae, H.O.; Yoo, J.C.; Kim, N.Y.; Kim, Y.C.; Ko, G.I.; Chung, H.T. Antiproliferative effects of alkaloids from Sedum sarmentosum on murine and human hepatoma cell lines. J. Ethnopharmacol. 2000, 70, 177–182. [Google Scholar] [CrossRef]
- Van Noordwijk, J.; Hollstein, U. The anthelminthic activity of pelletierine and isopelletierine. Acta Physiol. Pharmacol. Neerl. 1956, 5, 212–213. [Google Scholar] [PubMed]
- Van Noordwijk, J.; Mellink, J.J.; Visser, B.J.; Wisse, J.H. Synthesis and anthelmintic activity of isopelletierine and a series of side-chain homologues. Recl. Trav. Chim. Pays-Bas 2010, 82, 763–772. [Google Scholar] [CrossRef]
- Tripathi, S.M.; Singh, D.K. Molluscicidal activity of Punica granatum bark and Canna indica root. Braz. J. Med. Biol. Res. 2000, 33, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.M.; Singh, V.K.; Singh, S.; Singh, D.K. Enzyme inhibition by the molluscicidal agent Punica granatum Linn. Bark and Canna indica Linn. root. Phytother. Res. 2004, 18, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Chidiebere, M.A.; Ogukwe, C.E.; Oguzie, K.L.; Eneh, C.N.; Oguzie, E.E. Corrosion inhibition and adsorption behavior of Punica granatum extract on mild steel in acidic environments: Experimental and theoretical studies. Ind. Eng. Chem. Res. 2012, 51, 668–677. [Google Scholar] [CrossRef]
- Plowman, T. Botanical perspectives on coca. J. Psychedelic Drugs 1979, 11, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Plowman, T.; Hensold, N. Names, types, and distribution of neotropical species of Erythroxylum (Erythroxylaceae). Brittonia 2004, 56, 1–53. [Google Scholar] [CrossRef]
- Plowman, T. The ethnobotany of coca (Erythroxylum spp., Erythroxylaceae). In Ethnobotany in the Neotropics; Prance, G.T., Kallunki, J.A., Eds.; New York Botanical Garden: New York, NY, USA, 1984; pp. 62–111. [Google Scholar]
- Plowman, T. Amazonian coca. J. Ethnopharmacol. 1981, 3, 195–225. [Google Scholar] [CrossRef]
- Niemann, A. Ueber eine neue organische base in den cocablättern. Arch. Pharm. (Weinheim) 1860, 153, 291–308. [Google Scholar] [CrossRef]
- Freud, S. Ueber coca. ZentrBl. Ther. 1884, 2, 289–314. [Google Scholar]
- Plowman, T.; Rivier, L. Cocaine and cinnamoylcocaine content of Erythroxylum species. Ann. Bot. 1983, 51, 641–659. [Google Scholar]
- Naranjo, P. Social function of coca in pre-Columbian America. J. Ethnopharmacol. 1981, 3, 161–172. [Google Scholar] [CrossRef]
- Schmidt, E.; Henschke, H. Über die alkaloide der wurzel von Scopolia japonica. Arch. Pharm. (Weinheim) 1888, 226, 185–203. [Google Scholar] [CrossRef]
- Bisset, N.G. Arrow and dart poisons. J. Ethnopharmacol. 1989, 25, 1–41. [Google Scholar] [CrossRef]
- Schultes, R.E. Hallucinogenic plants. In A Golden Guide; Golden Press: New York, NY, USA, 1976. [Google Scholar]
- Hesse, G. Darstellung des atropins. Ann. Pharm. 1833, 5, 43–81. [Google Scholar]
- Mein. Darstellung des atropins in weissen krystallen. Ann. Pharm. 1833, 6, 67–72. [Google Scholar]
- Chilton, J.; Partridge, M.W. The partition chromatography of alkaloids. Part III—The alkaloids of Punica granatum. J. Pharm. Pharmacol. 1950, 2, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Hess, G.; Haiss, P.; Wistuba, D.; Siehl, H.-U.; Berger, S.; Sicker, D.; Zeller, K.-P. From the pomegranate tree to cyclooctatetraene: Pseudopelletierine. Chem. Unserer Zeit 2016, 50, 34–43. [Google Scholar]
- Khanna, K.L.; Schwarting, A.E.; Bobbitt, J.M. The occurrence of isopelletierine in Withania somnifera. J. Pharm. Sci. 1962, 51, 1194. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; T’Hart, H.; Stevens, J.F. Alkaloids of some Asian Sedum species. Phytochemistry 1996, 41, 1319–1324. [Google Scholar] [CrossRef]
- Chauhan, R.D.; Kanwar, K. Biotechnological advances in pomegranate (Punica granatum L.). In Vitro Cell. Dev. Biol. Plant 2012, 48, 579–594. [Google Scholar] [CrossRef]
- Brachet, A.; Muñoz, O.; Gupta, M.; Veuthey, J.-L.; Christen, P. Alkaloids of Erythroxylum lucidum stem-bark. Phytochemistry 1997, 46, 1439–1442. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Wink, M.; Botschen, F.; Gosmann, C.; Schäfer, H.; Waterman, P.G. Chemotaxonomy seen from a phylogenetic perspective and evolution of secondary metabolism. In Annual Plant Reviews Volume 40: Biochemistry of Plant Secondary Metabolism; Wink, M., Ed.; Wiley-Blackwell: Oxford, UK, 2010; Volume 40, pp. 364–433. [Google Scholar]
- Lazny, R.; Sienkiewicz, M.; Olenski, T.; Urbanczyk-Lipkowska, Z.; Kalicki, P. Approaches to the enantioselective synthesis of ferrugine and its analogues. Tetrahedron 2012, 68, 8236–8244. [Google Scholar] [CrossRef]
- Brock, A.; Herzfeld, T.; Paschke, R.; Koch, M.; Drager, B. Brassicaceae contain nortropane alkaloids. Phytochemistry 2006, 67, 2050–2057. [Google Scholar] [CrossRef] [PubMed]
- Magallon, S.; Castillo, S. Angiosperm diversification through time. Am. J. Bot. 2009, 96, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Evolution of secondary metabolites in legumes (Fabaceae). S. Afr. J. Bot. 2013, 89, 164–175. [Google Scholar] [CrossRef]
- Leete, E.; Marion, L.; Spenser, I.D. The biogenesis of alkaloids: 12. The mode of formation of the tropine base of hyoscyamine. Can. J. Chem. Rev. Can. Chim. 1954, 32, 1116–1123. [Google Scholar] [CrossRef]
- Liebisch, H.W.; Schütte, H.R. Zur biosynthese der tropanalkaloide. VIII. Vorstufen des pyrrolidinringes. Z. Pflanzenphysiol. 1967, 57, 434–439. [Google Scholar]
- Delauney, A.J.; Verma, D.P.S. Proline biosynthesis and osmoregulation in plants. Plant J. 1993, 4, 215–223. [Google Scholar] [CrossRef]
- Leete, E. Recent developments in the biosynthesis of the tropane alkaloids. Planta Med. 1990, 56, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Leete, E. Stereospecific incorporation of ornithine into tropine moiety of hyoscyamine. J. Am. Chem. Soc. 1962, 84, 55–57. [Google Scholar] [CrossRef]
- Leete, E. Biosynthesis of the pyrrolidine rings of cocaine and cuscohygrine from [5-14C]-labeled ornithine via a symmetrical intermediate. J. Am. Chem. Soc. 1982, 104, 1403–1408. [Google Scholar] [CrossRef]
- Docimo, T.; Reichelt, M.; Schneider, B.; Kai, M.; Kunert, G.; Gershenzon, J.; D’Auria, J.C. The first step in the biosynthesis of cocaine in Erythroxylum coca: The characterization of arginine and ornithine decarboxylases. Plant Mol. Biol. 2012, 78, 599–615. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, R.L.; Watson, M.B.; Galloway, G.L.; Yu, W. Molecular genetic analyses of plant polyamines. CRC Crit. Rev. Plant Sci. 1998, 17, 199–224. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, B.; Lu, B.; Kai, G.; Wang, Z.; Xia, Y.; Ding, R.; Zhang, H.; Sun, X.; Chen, W.; et al. Tropane alkaloids production in transgenic Hyoscyamus niger hairy root cultures over-expressing putrescine N-methyltransferase is methyl jasmonate-dependent. Planta 2007, 225, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Hibi, N.; Higashiguchi, S.; Hashimoto, T.; Yamada, Y. Gene expression in tobacco low-nicotine mutants. Plant Cell 1994, 6, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Galston, A.W.; Sawhney, R.K. Polyamines in plant physiology. Plant Physiol. 1990, 94, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Tamaki, K.; Suzuki, K.; Yamada, Y. Molecular cloning of plant spermidine synthases. Plant Cell Physiol. 1998, 39, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Yamada, Y.; Hashimoto, T. Expression of Atropa belladonna putrescine N-methyltransferase gene in root pericycle. Plant Cell Physiol. 1999, 40, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Hashimoto, T. Two tropinone reductases, that catalyze opposite stereospecific reductions in tropane alkaloid biosynthesis, are localized in plant root with different cell-specific patterns. Plant Cell Physiol. 1999, 40, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, J.; Facchini, P.J. Alkaloid biosynthesis: Metabolism and trafficking. Annu. Rev. Plant Biol. 2008, 59, 735–769. [Google Scholar] [CrossRef] [PubMed]
- Leete, E. Spermidine: An indirect precursor of the pyrrolidine rings of nicotine and nornicotine in Nicotiana glutinosa. Phytochemistry 1985, 24, 957–960. [Google Scholar] [CrossRef]
- Matsuzaki, R.; Fukui, T.; Sato, H.; Ozaki, Y.; Tanizawa, K. Generation of the topa quinone cofactor in bacterial monoamine oxidase by cupric ion-dependent autooxidation of a specific tyrosyl residue. FEBS Lett. 1994, 351, 360–364. [Google Scholar] [CrossRef]
- Heim, W.G.; Sykes, K.A.; Hildreth, S.B.; Sun, J.; Lu, R.H.; Jelesko, J.G. Cloning and characterization of a Nicotiana tabacum methylputrescine oxidase transcript. Phytochemistry 2007, 68, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Katoh, A.; Shoji, T.; Hashimoto, T. Molecular cloning of N-methylputrescine oxidase from tobacco. Plant Cell Physiol. 2007, 48, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Hoye, T.R.; Bjorklund, J.A.; Koltun, D.O.; Renner, M.K. N-methylputrescine oxidation during cocaine biosynthesis: Study of prochiral methylene hydrogen discrimination using the remote isotope method. Org. Lett. 2000, 2, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Romek, K.M.; Remaud, G.S.; Silvestre, V.; Paneth, P.; Robins, R.J. Non-statistical 13C fractionation distinguishes co-incident and divergent steps in the biosynthesis of the alkaloids nicotine and tropine. J. Biol. Chem. 2016, 291, 16620–16629. [Google Scholar] [CrossRef] [PubMed]
- Abraham, T.W.; Leete, E. New intermediate in the biosynthesis of the tropane alkaloids in Datura innoxia. J. Am. Chem. Soc. 1995, 117, 8100–8105. [Google Scholar] [CrossRef]
- Kaczkowski, J.; Schütte, H.R.; Mothes, K. Die rolle des acetats in der biosynthese der tropanalkaloide. Biochim. Biophys. Acta 1961, 46, 588–594. [Google Scholar] [CrossRef]
- Liebisch, H.W.; Peisker, K.; Radwan, A.S.; Schütte, H.R. Zur biosynthese der tropanalkaloide. XI. Die bildung der C3-brücke des tropins. Z. Pflanzenphysiol. 1972, 67, 1–9. [Google Scholar] [CrossRef]
- Robins, R.J.; Abraham, T.W.; Parr, A.J.; Eagles, J.; Walton, N.J. The biosynthesis of tropane alkaloids in Datura stramonium: The identity of the intermediates between N-methylpyrrolinium salt and tropinone. J. Am. Chem. Soc. 1997, 119, 10929–10934. [Google Scholar] [CrossRef]
- Leete, E.; Bjorklund, J.A.; Couladis, M.M.; Kim, S.H. Late intermediates in the biosynthesis of cocaine: 4-(1-Methyl-2-pyrrolidinyl)-3-oxobutanoate and methyl ecgonine. J. Am. Chem. Soc. 1991, 113, 9286–9292. [Google Scholar] [CrossRef]
- Hagel, J.M.; Facchini, P.J. Benzylisoquinoline alkaloid metabolism: A century of discovery and a brave new world. Plant Cell Physiol. 2013, 54, 647–672. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, A.J.; O’Hagan, D. Tropane alkaloid biosynthesis. A century old problem unresolved. Nat. Prod. Rep. 2001, 18, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.B.; Noel, J.P. The chalcone synthase superfamily of type III polyketide synthases. Nat. Prod. Rep. 2003, 20, 79–110. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C., Jr.; Vickery, C.R.; Burkart, M.D.; Noel, J.P. Confluence of structural and chemical biology: Plant polyketide synthases as biocatalysts for a bio-based future. Curr. Opin. Plant Biol. 2013, 16, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Jez, J.M.; Bowman, M.E.; Noel, J.P. Expanding the biosynthetic repertoire of plant type III polyketide synthases by altering starter molecule specificity. Proc. Natl. Acad. Sci. USA 2002, 99, 5319–5324. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Shimokawa, Y.; Matsui, T.; Kinjo, K.; Kato, R.; Noguchi, H.; Sugio, S.; Morita, H.; Abe, I. Cloning and structure-function analyses of quinolone- and acridone-producing novel type III polyketide synthases from Citrus microcarpa. J. Biol. Chem. 2013, 288, 28845–28858. [Google Scholar] [CrossRef] [PubMed]
- Resmi, M.S.; Verma, P.; Gokhale, R.S.; Soniya, E.V. Identification and characterization of a type III polyketide synthase involved in quinolone alkaloid biosynthesis from Aegle marmelos Correa. J. Biol. Chem. 2013, 288, 7271–7281. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, K.L.; Jornvall, H.; Persson, B.; Oppermann, U. Medium- and short-chain dehydrogenase/reductase gene and protein families: The SDR superfamily: Functional and structural diversity within a family of metabolic and regulatory enzymes. Cell. Mol. Life Sci. 2008, 65, 3895–3906. [Google Scholar] [CrossRef] [PubMed]
- Dräger, B. Tropinone reductases, enzymes at the branch point of tropane alkaloid metabolism. Phytochemistry 2006, 67, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Moummou, H.; Kallberg, Y.; Tonfack, L.B.; Persson, B.; van der Rest, B. The plant short-chain dehydrogenase (SDR) superfamily: Genome-wide inventory and diversification patterns. BMC Plant Biol. 2012, 12, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, K.; Kato, H.; Oda, J.; Yamada, Y.; Hashimoto, T. Site-directed mutagenesis of putative substrate-binding residues reveals a mechanism controlling the different stereospecificities of two tropinone reductases. J. Biol. Chem. 1999, 274, 16563–16568. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Hashimoto, T.; Yamada, Y. Two tropinone reductases with different stereospecificities are short-chain dehydrogenases evolved from a common ancestor. Proc. Natl. Acad. Sci. USA 1993, 90, 9591–9595. [Google Scholar] [CrossRef] [PubMed]
- Jez, J.M.; Bennett, M.J.; Schlegel, B.P.; Lewis, M.; Penning, T.M. Comparative anatomy of the aldo-keto reductase superfamily. Biochem. J. 1997, 326, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.; Clouet, D.H.; Misra, A.L.; Mule, S. Cocaine and metabolites—Relationship between pharmacological activity and inhibitory action on dopamine uptake into striatal synaptosomes. Prog. Neuropsychopharmacol. 1977, 1, 265–269. [Google Scholar] [CrossRef]
- Bjorklund, J.A.; Leete, E. Biosynthesis of the benzoyl moiety of cocaine from cinnamic acid via (R)-(+)-3-hydroxy-3-phenylpropanoic acid. Phytochemistry 1992, 31, 3883–3887. [Google Scholar] [CrossRef]
- Leete, E.; Bjorklund, J.A.; Kim, S.H. The biosynthesis of the benzoyl moiety of cocaine. Phytochemistry 1988, 27, 2553–2556. [Google Scholar] [CrossRef]
- D’Auria, J.C. Acyltransferases in plants: A good time to be BAHD. Curr. Opin. Plant Biol. 2006, 9, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Rabot, S.; Peerless, A.C.J.; Robins, R.J. Tigloyl-CoA: Pseudotropine acyl transferase—An enzyme of tropane alkaloid biosynthesis. Phytochemistry 1995, 39, 315–322. [Google Scholar] [CrossRef]
- Schmidt, G.W.; Jirschitzka, J.; Porta, T.; Reichelt, M.; Luck, K.; Pardo Torre, J.C.; Dolke, F.; Varesio, E.; Hopfgartner, G.; Gershenzon, J.; et al. The last step in cocaine biosynthesis is catalyzed by a BAHD acyltransferase. Plant Physiol. 2015, 167, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Torre, J.C.; Schmidt, G.W.; Paetz, C.; Reichelt, M.; Schneider, B.; Gershenzon, J.; D’Auria, J.C. The biosynthesis of hydroxycinnamoyl quinate esters and their role in the storage of cocaine in Erythroxylum coca. Phytochemistry 2013, 91, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Leete, E.; Kowanko, N.; Newmark, R.A. Use of carbon-13 nuclear magnetic-resonance to establish that biosynthesis of tropic acid involves an intramolecular rearrangement of phenylalanine. J. Am. Chem. Soc. 1975, 97, 6826–6830. [Google Scholar] [CrossRef] [PubMed]
- Ansarin, M.; Woolley, J.G. The rearrangement of phenyllactate in the biosynthesis of tropic acid. Phytochemistry 1994, 35, 935–939. [Google Scholar] [CrossRef]
- Robins, R.J.; Bachmann, P.; Woolley, J.G. Biosynthesis of hyoscyamine involves an intramolecular rearrangement of littorine. J. Chem. Soc. Perkin Trans. 1994, 25, 615–619. [Google Scholar] [CrossRef]
- Chesters, N.C.J.E.; O’Hagan, D.; Robins, R.J. The biosynthesis of tropic acid: The (R)-d-phenyllactyl moiety is processed by the mutase involved in hyoscyamine biosynthesis in Datura stramonium. J. Chem. Soc. Chem. Commun. 1995, 2, 127–128. [Google Scholar] [CrossRef]
- Robins, R.J.; Chesters, N.C.J.E.; O’Hagan, D.; Parr, A.J.; Walton, N.J.; Woolley, J.G. The biosynthesis of hyoscyamine: The process by which littorine rearranges to hyoscyamine. J. Chem. Soc. Perkin Trans. 1995, 4, 481–485. [Google Scholar] [CrossRef]
- Sandala, G.M.; Smith, D.M.; Radom, L. The carbon-skeleton rearrangement in tropane alkaloid biosynthesis. J. Am. Chem. Soc. 2008, 130, 10684–10690. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Reed, D.W.; Liu, E.W.; Nowak, J.; Pelcher, L.E.; Page, J.E.; Covello, P.S. Functional genomic analysis of alkaloid biosynthesis in Hyoscyamus niger reveals a cytochrome p450 involved in littorine rearrangement. Chem. Biol. 2006, 13, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Nasomjai, P.; Reed, D.W.; Tozer, D.J.; Peach, M.J.G.; Slawin, A.M.Z.; Covello, P.S.; O’Hagan, D. Mechanistic insights into the cytochrome p450-mediated oxidation and rearrangement of littorine in tropane alkaloid biosynthesis. ChemBioChem 2009, 10, 2382–2393. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.; Jamali, A.; Lanoue, A.; Gontier, E.; Dauwe, R. Unravelling the architecture and dynamics of tropane alkaloid biosynthesis pathways using metabolite correlation networks. Phytochemistry 2015, 116, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Yamada, Y. Hyoscyamine 6b-hydroxylase, a 2-oxoglutarate-dependent dioxygenase, in alkaloid-producing root cultures. Plant Physiol. 1986, 81, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Matsuda, J.; Yamada, Y. Two-step epoxidation of hyoscyamine to scopolamine is catalyzed by bifunctional hyoscyamine 6b-hydroxylase. FEBS Lett. 1993, 329, 35–39. [Google Scholar] [CrossRef]
- Hashimoto, T.; Hayashi, A.; Amano, Y.; Kohno, J.; Iwanari, H.; Usuda, S.; Yamada, Y. Hyoscyamine 6b-hydroxylase, an enzyme involved in tropane alkaloid biosynthesis, is localized at the pericycle of the root. J. Biol. Chem. 1991, 266, 4648–4653. [Google Scholar] [PubMed]
- Beyerman, H.C.; Maat, L. Resolution of isopelletierine: A second synthesis of pelletierine. Recl. Trav. Chim. Pays-Bas 1965, 84, 385–388. [Google Scholar] [CrossRef]
- Leistner, E.; Gupta, R.N.; Spenser, I.D. A general method for the determination of precursor configuration in biosynthetic precursor-product relationships. Derivation of pipecolic acid from d-lysine, and of piperidine alkaloids from l-lysine. J. Am. Chem. Soc. 1973, 95, 4040–4047. [Google Scholar] [CrossRef] [PubMed]
- Hemscheidt, T.; Spenser, I.D. Biosynthesis of N-methylpelletierine: Vindication of a classical biogenetic concept. J. Am. Chem. Soc. 1990, 112, 6360–6363. [Google Scholar] [CrossRef]
- Gupta, R.N.; Spenser, I.D. Biosynthesis of N-methylpelletierine. Phytochemistry 1969, 8, 1937–1944. [Google Scholar] [CrossRef]
- Keogh, M.F.; Odonovan, D.G. Biosynthesis of some alkaloids of Punica granatum and Withania somnifera. J. Chem. Soc. C 1970, 13, 1792–1797. [Google Scholar] [CrossRef]
- O’Donovan, D.G.; Keogh, M.F. Biosynthesis of piperidine alkaloids. Tetrahedron Lett. 1968, 3, 265–267. [Google Scholar] [CrossRef]
- Gupta, R.N.; Spenser, I.D. Biosynthesis of the piperidine alkaloids. Origin of the piperidine nucleus of N-methylisopelletierine. Chem. Commun. 1968, 2, 85–86. [Google Scholar] [CrossRef]
- Liebisch, H.W.; Marekov, N.; Schutte, H.R. Biosynthesis of alkaloids from Punica granatum. Z. Naturforsch. B 1968, 23, 1116–1117. [Google Scholar] [CrossRef] [PubMed]
- Leistner, E.; Spenser, I.D. Biosynthesis of the piperidine nucleus. Incorporation of chirally labeled cadaverine-1–3H. J. Am. Chem. Soc. 1973, 95, 4715–4725. [Google Scholar] [CrossRef] [PubMed]
- Shorrosh, B.S.; Dixon, R.A.; Ohlrogge, J.B. Molecular cloning, characterization, and elicitation of acetyl-CoA carboxylase from alfalfa. Proc. Natl. Acad. Sci. USA 1994, 91, 4323–4327. [Google Scholar] [CrossRef] [PubMed]
- Bunsupa, S.; Komastsu, K.; Nakabayashi, R.; Saito, K.; Yamasaki, M. Revisiting anabasine biosynthesis in tobacco hairy roots expressing plant lysine decarboxylase gene by using 15N-labeled lysine. Phytochemistry 2014, 31, 511–588. [Google Scholar]
- Riechers, D.E.; Timko, M.P. Structure and expression of the gene family encoding putrescine N-methyltransferase in Nicotiana tabacum: New clues to the evolutionary origin of cultivated tobacco. Plant Mol. Biol. 1999, 41, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Winz, R.A.; Baldwin, I.T. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. IV. Insect-induced ethylene reduces jasmonate-induced nicotine accumulation by regulating putrescine N-methyltransferase transcripts. Plant Physiol. 2001, 125, 2189–2202. [Google Scholar] [PubMed]
- Cona, A.; Rea, G.; Angelini, R.; Federico, R.; Tavladoraki, P. Functions of amine oxidases in plant development and defence. Trends Plant Sci. 2006, 11, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Pontvianne, F.; Blevins, T.; Pikaard, C.S. Arabidopsis histone lysine methyltransferases. Adv. Bot. Res. 2010, 53, 1–22. [Google Scholar] [PubMed]
- Leete, E. Biosynthesis of cocaine and cuscohygrine in Erythroxylon coca. J. Chem. Soc. Chem. Commun. 1980, 22, 1170–1171. [Google Scholar] [CrossRef]
- Yu, G.; Nguyen, T.T.H.; Guo, Y.; Schauvinhold, I.; Auldridge, M.E.; Bhuiyan, N.; Ben-Israel, I.; Iijima, Y.; Fridman, E.; Noel, J.P.; et al. Enzymatic functions of wild tomato methylketone synthases 1 and 2. Plant Physiol. 2010, 154, 67–77. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Who Model List of Essential Medicines, 17th List (March 2011); World Health Organization: Geneva, Switzerland, 2011; p. 41. [Google Scholar]
- Ullrich, S.F.; Hagels, H.; Kayser, O. Scopolamine: A journey from the field to clinics. Phytochem. Rev. 2016. [Google Scholar] [CrossRef]
- Wang, X.; Chen, M.; Yang, C.; Liu, X.; Zhang, L.; Lan, X.; Tang, K.; Liao, Z. Enhancing the scopolamine production in transgenic plants of Atropa belladonna by overexpressing pmt and h6h genes. Physiol. Plant. 2011, 143, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.G.; Xia, X.X.; Lee, S.Y. Metabolic engineering of Escherichia coli for the production of putrescine: A four carbon diamine. Biotechnol. Bioeng. 2009, 104, 651–662. [Google Scholar] [PubMed]
- Cardillo, A.B.; Giulietti, A.M.; Palazon, J.; Bonfill, M. Influence of hairy root ecotypes on production of tropane alkaloids in Brugmansia candida. Plant Cell Tissue Organ Cult. 2013, 114, 305–312. [Google Scholar] [CrossRef]
- Richter, U.; Rothe, G.; Fabian, A.K.; Rahfeld, B.; Drager, B. Overexpression of tropinone reductases alters alkaloid composition in Atropa belladonna root cultures. J. Exp. Bot. 2005, 56, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Kai, G.; Yang, S.; Luo, X.; Zhou, W.; Fu, X.; Zhang, A.; Zhang, Y.; Xiao, J. Co-expression of AaPMT and AaTRI effectively enhances the yields of tropane alkaloids in Anisodus acutangulus hairy roots. BMC Biotechnol. 2011, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.-H.; Pan, X.-C.; Qiang, W.; Liao, Z.-H. Improvement of biosynthesis of tropane alkaloids in Anisodus acutangulus by Co-transformed PMT and H6H. Plant Omics 2014, 36, 21–27. [Google Scholar]
- Rothe, G.; Hachiya, A.; Yamada, Y.; Hashimoto, T.; Drager, B. Alkaloids in plants and root cultures of Atropa belladonna overexpressing putrescine N-methyltransferase. J. Exp. Bot. 2003, 54, 2065–2070. [Google Scholar] [CrossRef] [PubMed]
- Moyano, E.; Jouhikainen, K.; Tammela, P.; Palazón, J.; Cusidó, R.M.; Piñol, M.T.; Teeri, T.H.; Oksman-Caldentey, K.-M. Effect of pmt gene overexpression on tropane alkaloid production in transformed root cultures of Datura metel and Hyoscyamus muticus. J. Exp. Bot. 2003, 54, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.J.; Hashimoto, T.; Yamada, Y. Metabolic engineering of medicinal plants: Transgenic Atropa belladonna with an improved alkaloid composition. Proc. Natl. Acad. Sci. USA 1992, 89, 11799–11803. [Google Scholar] [CrossRef] [PubMed]
- Jouhikainen, K.; Lindgren, L.; Jokelainen, T.; Hiltunen, R.; Teeri, T.H.; Oksman-Caldentey, K.-M. Enhancement of scopolamine production in Hyoscyamus muticus L. hairy root cultures by genetic engineering. Planta 1999, 208, 545–551. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, R.; Chai, Y.; Bonfill, M.; Moyano, E.; Oksman-Caldentey, K.-M.; Xu, T.; Pi, Y.; Wang, Z.; Zhang, H.; et al. Engineering tropane biosynthetic pathway in Hyoscyamus niger hairy root cultures. Proc. Natl. Acad. Sci. USA 2004, 101, 6786–6791. [Google Scholar] [CrossRef] [PubMed]
- Archana Giri, M.L.N. Transgenic hairy roots: Recent trends and applications. Biotechnol. Adv. 2000, 18, 1–22. [Google Scholar] [CrossRef]
- Wu, J.Y.; Ng, J.; Shi, M.; Wu, S.J. Enhanced secondary metabolite (tanshinone) production of Salvia miltiorrhiza hairy roots in a novel root-bacteria coculture process. Appl. Microbiol. Biotechnol. 2007, 77, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Kai, G.; Yang, S.; Zhang, Y.; Luo, X.; Fu, X.; Zhang, A.; Xiao, J. Effects of different elicitors on yield of tropane alkaloids in hairy roots of Anisodus acutangulus. Mol. Biol. Rep. 2012, 39, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Amdoun, R.; Khelifi, L.; Khelifi-Slaoui, M.; Amroune, S.; Benyoussef, E.H.; Thi, D.V.; Assaf-Ducrocq, C.; Gontier, E. Influence of minerals and elicitation on Datura stramonium L. tropane alkaloid production: Modelization of the in vitro biochemical response. Plant Sci. 2009, 177, 81–87. [Google Scholar] [CrossRef]
- Zhao, X.C.; Qu, X.; Mathews, D.E.; Schaller, G.E. Effect of ethylene pathway mutations upon expression of the ethylene receptor ETR1 from Arabidopsis. Plant Physiol. 2002, 130, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Parvatam, G.; Ravishankar, G.A. AgNO3—A potential regulator of ethylene activity and plant growth modulator. Electron. J. Biotechnol. 2009, 12, 1–15. [Google Scholar] [CrossRef]
- Anantasaran, J.; Kanchanapoom, K. Influence of medium formula and silver nitrate on in vitro plant regeneration of Zinnia cultivars. Songklanakarin J. Sci. Technol. 2008, 30, 1–6. [Google Scholar]
- Angelova, Z.; Georgiev, S.; Roos, W. Elicitation of plants. Biotechnol. Biotechnol. Equip. 2006, 20, 72–83. [Google Scholar] [CrossRef]
- Pitta–Alvarez, S.I.; Spollansky, T.C.; Giulietti, A.M. The influence of different biotic and abiotic elicitors on the production and profile of tropane alkaloids in hairy root cultures of Brugmansia candida. Enzym. Microb. Technol. 2000, 26, 252–258. [Google Scholar] [CrossRef]
- Sahandi, S.; Sorooshzadeh, A.; Rezazadeh, H.S.; Naghdlbadl, H.A. Effect of nano silver and silver nitrate on seed yield of borage. J. Med. Plant. Res. 2011, 5, 706–710. [Google Scholar]
- Lee, W.M.; Kwak, J.I.; An, Y.J. Effect of silver nanoparticles in crop plants Phaseolus radiatus and Sorghum bicolor: Media effect on phytotoxicity. Chemosphere 2012, 86, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.-G.; Xia, X.-X.; Lee, S.Y. Metabolic engineering of Escherichia coli for the production of cadaverine: A five carbon diamine. Biotechnol. Bioeng. 2011, 108, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Lili, M.; Wang, Y.; Chen, M.; Lan, X.; Wu, N.; Liao, Z. Effects of acetylsalicylic acid and UV-B on gene expression and tropane alkaloid biosynthesis in hairy root cultures of Anisodus luridus. Plant Cell Tissue Organ Cult. 2014, 117, 483–490. [Google Scholar] [CrossRef]
- Docimo, T.; Davis, A.J.; Luck, K.; Fellenberg, C.; Reichelt, M.; Phillips, M.; Gershenzon, J.; D’Auria, J.C. Influence of medium and elicitors on the production of cocaine, amino acids and phytohormones by Erythroxylum coca calli. Plant Cell Tissue Organ Cult. 2014, 120, 1061–1075. [Google Scholar] [CrossRef]
- Coste, A.; Vlase, L.; Halmagyi, A.; Deliu, C.; Coldea, G. Effects of plant growth regulators and elicitors on production of secondary metabolites in shoot cultures of Hypericum hirsutum and Hypericum maculatum. Plant Cell Tissue Organ Cult. 2011, 106, 279–288. [Google Scholar] [CrossRef]
- Wurtzel, E.T.; Kutchan, T.M. Plant metabolism, the diverse chemistry set of the future. Science 2016, 353, 1232–1236. [Google Scholar] [CrossRef] [PubMed]








| Species Studied | Radiolabeled Compound | Where Label Was Found | Reference |
|---|---|---|---|
| Punica granatum | 1-14C-acetate | N-methyl isopelletierine | [119] |
| Punica granatum | 2-14C-lysine | N-methyl isopelletierine | [119] |
| Withania somnifera | 2-14C-lysine | anaferine | [119] |
| Punica granatum | [1-14C] acetate | Isopelletierine, N-methylisopelletierine, and pseudopelletierine | [118] |
| Withania somnifera | [1-14C] acetate | anaferine | [118] |
| Punica granatum | dl-[2-14C] lysine | N-methylisopelletierine asymmetrically | [118] |
| Withania somnifera | dl-[2-14C] lysine | Anaferine asymmetrically | [118] |
| Punica granatum | [N-methyl-14C, 814C] methylisopelletierine | pseudopelletierine | [118] |
| Withania somnifera | [8-14C] isopelletierine | anaferine | [118] |
| Punica granatum | [1,515C] cadaverine and [3H] cadaverine | N-methylisopelletierine and pseudopelletierine nonrandomly | [121] |
| Punica granatum | [14C] methionine | N-methylisopelletierine and pseudopelletierine | [121] |
| Sedum sarmentosum | Sodium [1,2,3,4-13C4] acetate and sodium [1,2-13C2] acetate | N-methylpelletierine | [116] |
| Sedum acre and Sedum sarmentosum | dl-[6-14C] lysine, l-[4,5-8H2] lysine, and d-[6-14C] lysine | Only l enantiomer of lysine was incorporated into N-methylpelletierine. | [115] |
| Nicotiana glauca | dl-[6-14C] lysine, l-[4,5-8H2] lysine, and d-[6-14C] lysine | Only l enantiomer of lysine was incorporated into anabasine. | [115] |
| Sedum sarmentosum | [6-14C] lysine | N-methylisopelletierine | [120] |
| Sedum sarmentosum | 6-14C-dl-lysine | N-methylpelletierine asymmetrically | [117] |
| Sedum sarmentosum | 4,5-3H2,6-14C-dl-lysine | N-methylpelletierine | [117] |
| Species | Target Compound | Gene/Genes Modified | Effect | Reference |
|---|---|---|---|---|
| Atropa belladonna | Scopolamine | NtPMT & HnH6H | Increased scopolamine content | [11] |
| Atropa belladonna | Hyoscyamine & scopolamine | rolC, pmt, & h6h | Increased hyoscyamine content & increased scopolamine content | [133] |
| Escherichia coli | Putrescine | Multiple | Increased putrescine production | [134] |
| Brugmansia condida | Polyamines (putrescine) | rolC | Polyamine accumulation, improve hairy root growth | [135] |
| Atropa belladonna | Pseudotropine/Tropine | tr-1/tr-2 | Higher enzyme activity & increase in pseudotropine/tropine | [136] |
| Anisodus acutangulus | TAs | pmt & tr-1 | Increased TA levels with hyoscyamine being major alkaloid | [137] |
| Species | Target Compound | Elicitor | Effect | Reference |
|---|---|---|---|---|
| Anisodus luridus | Scopolamine | Acetylsalicylic acid (ASA) | Increased scopolamine content | [156] |
| Anisodus luridus | Scopolamine | Ultraviolet ray-B (UV-B) | Increased scopolamine content | [156] |
| Datura metel | Atropine | Staphylococcus aureus | Decreased atropine content, increased root biomass | [10] |
| Datura metel | Atropine | Bacillus cereus | Decreased atropine content, increased root biomass | [10] |
| Datura metel | Atropine | Silver nitrate | Decreased atropine content, increased root biomass | [10] |
| Datura metel | Atropine | Nanosilver | Increased atropine content, increased root biomass | [10] |
| Datura innoxia | Hyoscyamine | Agrobacterium rhizogenes | Increased hyoscyamine content, increased root biomass | [110] |
| Erythroxylum coca | Cocaine | Anderson rhododendron medium (ARM) | Increased cocaine content in calli | [157] |
| Erythroxylum coca | Chlorogenic acid (CGA) | Salicylic acid | Decreased CGA content | [157] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.; Estrada, O.; Chavez, B.; Stewart, C.; D’Auria, J.C. Tropane and Granatane Alkaloid Biosynthesis: A Systematic Analysis. Molecules 2016, 21, 1510. https://doi.org/10.3390/molecules21111510
Kim N, Estrada O, Chavez B, Stewart C, D’Auria JC. Tropane and Granatane Alkaloid Biosynthesis: A Systematic Analysis. Molecules. 2016; 21(11):1510. https://doi.org/10.3390/molecules21111510
Chicago/Turabian StyleKim, Neill, Olga Estrada, Benjamin Chavez, Charles Stewart, and John C. D’Auria. 2016. "Tropane and Granatane Alkaloid Biosynthesis: A Systematic Analysis" Molecules 21, no. 11: 1510. https://doi.org/10.3390/molecules21111510