A Metal-Free Regioselective Multicomponent Approach for the Synthesis of Free Radical Scavenging Pyrimido-Fused Indazoles and Their Fluorescence Studies
Abstract
:1. Introduction
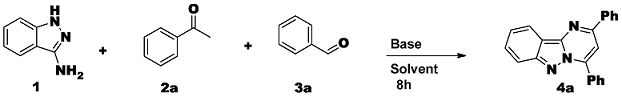
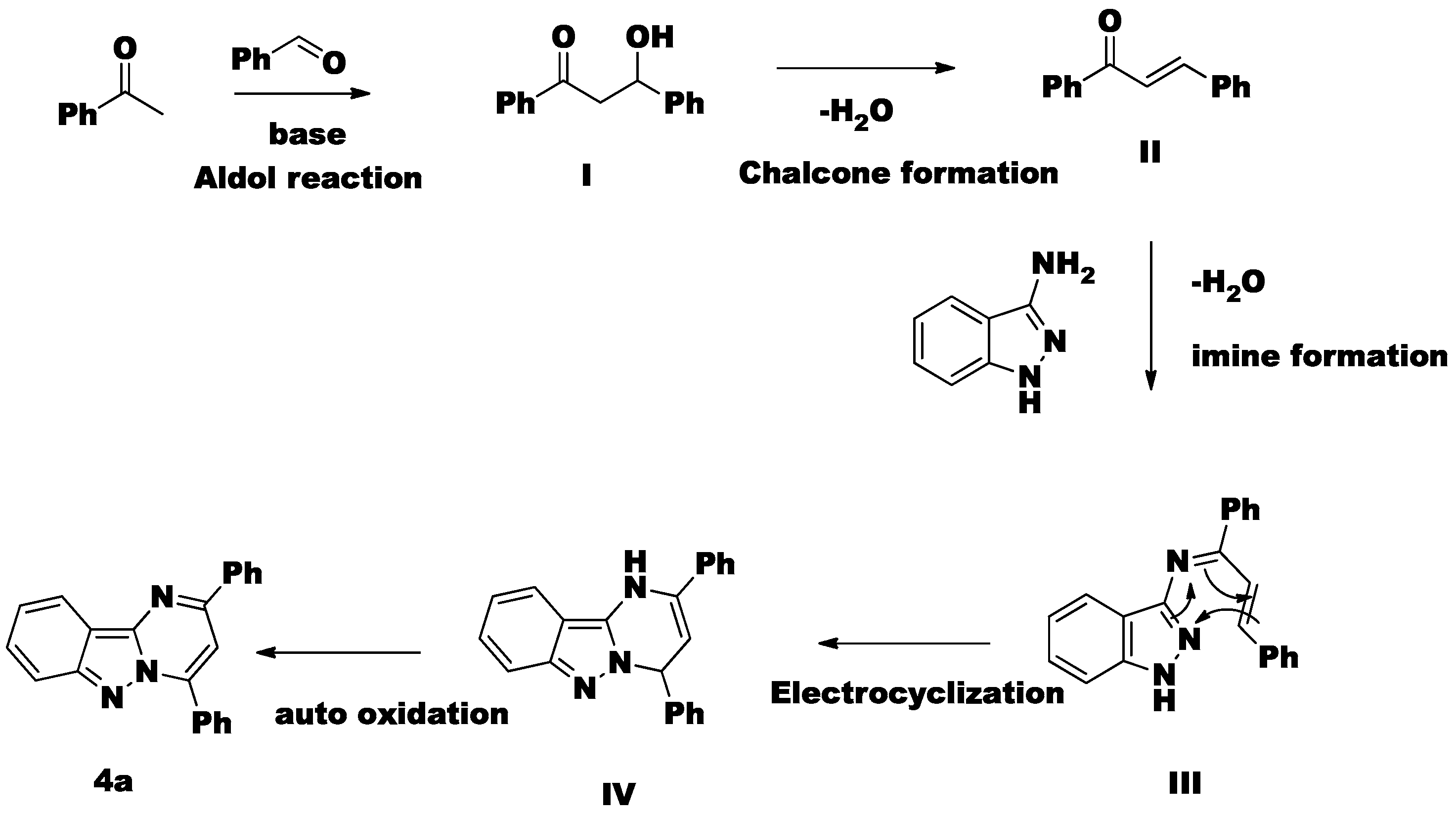
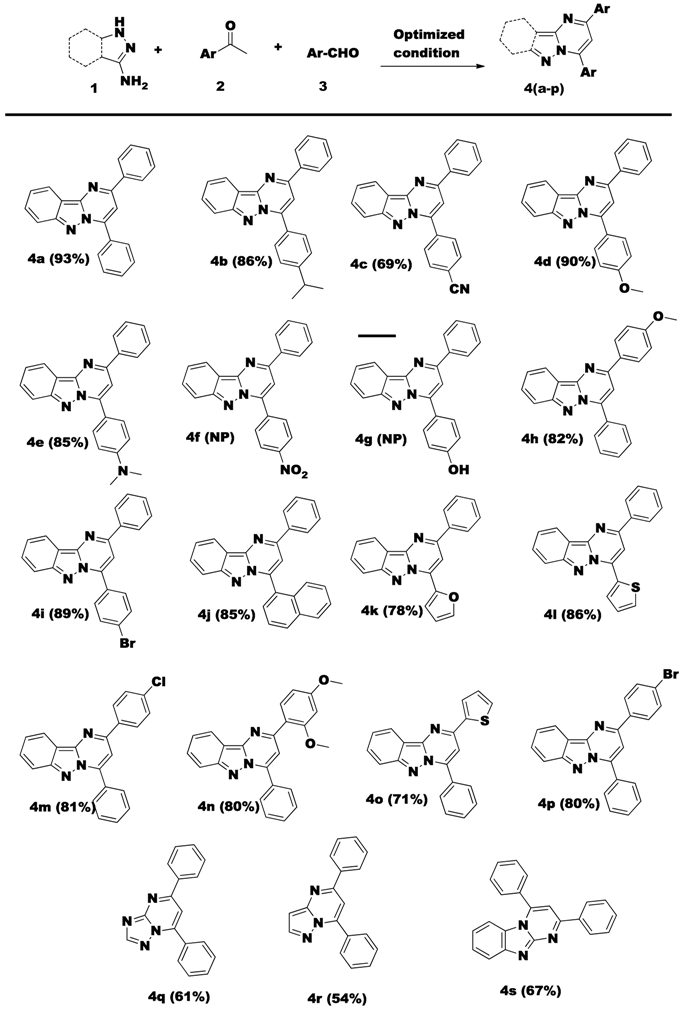
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of 2,4-Diphenylpyrimido[1,2-b]indazoles 4(a–s) via Metal Free Conditions
3.3. Characterization Data
3.4. Experimental Design and Mathematical Model
3.5. Antioxidant Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fiorot, R.G.; Filho, J.F.A.; Pereria, T.M.C.; Lacerda, V., Jr.; Santos, R.B.D.; Romao, W.; Greco, S.J.; Keating, T.A. A simple and convenient method for synthesis of new aminonaphthoquinones derived from lawsone by catalytic multicomponent Mannich reaction. Tetrahedron Lett. 2014, 55, 4373–4377. [Google Scholar] [CrossRef]
- Rahmati, A.; Ahmadi, S.; Varzaneh, M.A. One-pot synthesis of 1,2,4,5-tetrahydro-2,4-dioxobenzo[b]-[1,4]diazepine and malonamide derivatives using multi-component reactions. Tetrahedron 2014, 70, 9512–9521. [Google Scholar] [CrossRef]
- Gore, R.P.; Rajput, A.P. A review on recent progress in multicomponent reactions of pyrimidines synthesis. Drug Invent. Today 2013, 2, 148–152. [Google Scholar] [CrossRef]
- Terzidis, M.A.; Drosos, N.M.; Kasapidou, P.M.; Stephanatou, J.S.; Tsiaras, V.G.; Buth, G.; Tsoleridis, C.A.; Kostakis, G.E. Chromeno[2,3-c]pyrroles by one-pot multicomponent domino addition–amination reaction. Tetrahedron Lett. 2014, 55, 5601–5604. [Google Scholar] [CrossRef]
- Feng, H.; Zhao, P.; Sun, Z. CuI/CuBr2-catalyzed decarboxylative/A3 reaction of propiolic acids for the facile synthesis of 1,4-diheterocycle-2-butynes. Tetrahedron Lett. 2015, 56, 5676–5680. [Google Scholar] [CrossRef]
- Roopan, S.M.; Bharathi, A.; Palaniraja, J.; Gengan, R.M.; Anand, K. Unexpected regiospecific Michael addition product: Synthesis of 5,6-dihydrobenzo[1,7]phenanthrolines. RSC Adv. 2015, 48, 38640–38645. [Google Scholar] [CrossRef]
- Subhashini, R.; Roopan, S.M.; Khan, F.R.N. Synthesis and free radical scavenging property of some quinoline derivatives. J. Chil. Chem. Soc. 2010, 55, 317–319. [Google Scholar] [CrossRef]
- Wang, J.; Shen, Q.; Zhang, J.; Song, G. Metal-free multicomponent coupling reaction of aliphatic amines, formaldehyde, organoboronic acids, and propiolic acids for the synthesis of diverse propargylamines. Tetrahedron Lett. 2015, 56, 903–906. [Google Scholar] [CrossRef]
- Bondzic, B.P. Rh catalyzed multicomponent tandem and one-pot reactions under hydroformylation conditions. J. Mol. Catal. A Chem. 2015, 408, 310–334. [Google Scholar] [CrossRef]
- Devi, N.; Rawal, R.K.; Singh, V. Diversity-oriented synthesis of fused-imidazole derivatives via Groebke–Blackburn–Bienayme reaction: A review. Tetrahedron 2015, 71, 183–232. [Google Scholar] [CrossRef]
- Ugi, I. Recent progress in the chemistry of multicomponent reactions. Pure Appl. Chem. 2001, 73, 187–191. [Google Scholar] [CrossRef]
- Surendra, K.; Krishnaveni, N.S.; Mahesh, A.; Rao, K.R. Supramolecular Catalysis of Strecker Reaction in Water under Neutral Conditions in the Presence of β-Cyclodextrin. J. Org. Chem. 2006, 71, 2532–2534. [Google Scholar] [CrossRef] [PubMed]
- Janardhan, B.; Rajitha, B.; Crooks, P.A. Poly(4-vinylpyridinium)hydrogen sulfate: An efficient heterogeneous catalyst for the one-pot synthesis of polyhydroquinolines via unsymmetrical Hantzsch reaction in aqueous medium. J. Saudi Chem. Soc. 2014, 18, 722–727. [Google Scholar] [CrossRef]
- Zamani, F.; Izadi, E. Synthesis and characterization of sulfonated-phenylacetic acid coated Fe3O4 nanoparticles as a novel acid magnetic catalyst for Biginelli reaction. Catal. Commun. 2013, 42, 104–108. [Google Scholar] [CrossRef]
- Dongare, S.B.; Chavan, H.V.; Bhale, P.S.; Mule, Y.B.; Kotmale, A.S.; Bandgar, B.P. A catalyst- and solvent-free multicomponent synthesis of 7-azagramine analogues via a Mannich type reaction. Chin. Chem. Lett. 2016, 27, 99–103. [Google Scholar] [CrossRef]
- Wang, H.; Deng, T.; Cai, C. Fluorousbis(oxazolines) ligand: Synthesis and application in Kabachnik-Fields reaction. J. Flour. Chem. 2014, 168, 144–150. [Google Scholar] [CrossRef]
- Montagne, C.; Montagne, M.; Shipman, M.; Shipman, M. Modified Bucherer-Bergs Reaction for the One-Pot Synthesis of 5,5′-Disubstituted Hydantoins from Nitriles and Organometallic Reagents. Synlett 2006, 14, 2203–2206. [Google Scholar] [CrossRef]
- Han, Y.; Tang, W.Q.; Yan, C.G. Gewald-type reaction of double activated 2,3-diarylcyclopropanes with elemental sulfur for synthesis of polysubstituted 2-aminothiophenes. Tetrahedron 2014, 55, 1441–1443. [Google Scholar] [CrossRef]
- Salim, S.D.; Pathare, S.P.; Akamanchi, K.G. Sulfated tungstate: A green catalyst for synthesis of thiomorpholides via Willgerodt–Kindler reaction. Catal. Commun. 2011, 13, 78–81. [Google Scholar] [CrossRef]
- Borah, P.; Boarh, J.M.; Chowdhury, P. Microwave (MW) irradiated Ugi four-component reaction (Ugi-4CR): Expedited synthesis of steroid–amino acid conjugates—A novel class of hybrid compounds. Steroids 2015, 98, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Varyani, M.; Khatri, P.K.; Jain, S.L. Amino acid ionic liquid bound copper Schiff base catalyzed highly efficient three component A3-coupling reaction. Catal. Commun. 2016, 77, 113–117. [Google Scholar] [CrossRef]
- Roopan, S.M.; Palaniraja, J. Synthetic journey towards transition metal-free arylations. Res. Chem. Intermed. 2015, 41, 8111–8146. [Google Scholar] [CrossRef]
- Palaniraja, J.; Roopan, S.M. UV-light induced domino type reactions: Synthesis and photophysical properties of unreported nitrogen ring junction quinazolines. RSC Adv. 2015, 5, 37415–37423. [Google Scholar] [CrossRef]
- Palaniraja, J.; Roopan, S.M. Iodine-mediated synthesis of indazolo-quinazolinones via a multi-component reaction. RSC Adv. 2015, 12, 8640–8646. [Google Scholar] [CrossRef]
- Palaniraja, J.; Roopan, S.M.; Rayalu, G.M. One-pot synthesis of highly functionalized pyrimido[1,2-b]indazoles via 6-endo-dig cyclization. RSC Adv. 2016, 6, 24610–24616. [Google Scholar] [CrossRef]
- Noshadia, I.; Amina, N.A.S.; Parnasb, R.S. Continuous production of biodiesel from waste cooking oil in a reactive distillation column catalyzed by solid heteropolyacid: Optimization using response surface methodology (RSM). Fuel 2012, 94, 156–164. [Google Scholar] [CrossRef]
- Wie, L.; Li, X.; Yanxun, L.; Tingliang, L.; Guoji, L. Process Variables of the Esterification Reaction of 3-Pentadecylphenol and Acryloyl Chloride Catalyzed by an Ionic Liquid. Chem. Eng. Technol. 2013, 36, 559–566. [Google Scholar] [CrossRef]
- Yin, P.; Chen, L.; Wang, Z.; Qu, R.; Liu, X.; Xu, Q.; Ren, S. Biodiesel production from esterification of oleic acid over aminophosphonic acid resin D418. Fuel 2012, 102, 499–505. [Google Scholar] [CrossRef]
- Yin, P.; Chen, W.; Liu, W.; Chen, H.; Qu, R.; Liu, X.; Tang, Q.; Xu, Q. Efficient bifunctional catalyst lipase/organophosphonic acid-functionalized silica for biodiesel synthesis by esterification of oleic acid with ethanol. Bioresour. Technol. 2013, 140, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Jinyu, X.; Weimin, L. Response surface design of solid waste based geopolymer. RSC Adv. 2015, 5, 1598–1604. [Google Scholar] [CrossRef]
- Divsar, F.; Habibzadeh, K.; Shariati, S.; Shahriarinour, M. Aptamer conjugated silver nanoparticles for the colorimetric detection of arsenic ions using response surface methodology. Anal. Methods 2015, 7, 4568–4576. [Google Scholar] [CrossRef]
- Ye, M.; Edmunds, A.J.F.; Morris, J.A.; Sale, D.; Zhang, Y.; Yu, J.Q. A Robust Protocol for Pd(II)-catalyzed C-3 Arylation of (1H) Indazoles and Pyrazoles: Total Synthesis of NigellidineHydrobromide. Chem. Sci. 2013, 4, 2374–2379. [Google Scholar] [CrossRef] [PubMed]
- Hemealtha, K.; Madhumitha, G.; Vasavi, C.S.; Munusami, P. 2,3-Dihydroquinazolin-4(1H)-ones: Visible light mediated synthesis, solvatochromism and biological activity. J. Photochem. Photobiol. B 2013, 143, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, S.; Roopan, S.M.; Al-Dhabi, N.A.; Suthindhiran, K.; Gargi, S.; Arasu, M.A. 1,2,4-Triazolo-quinazoline-thiones: Non-conventional synthetic approach, study of solvatochromism and antioxidant assessment. J. Photochem. Photobiol. B 2016, 162, 232–239. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.




| Reaction | Variables | Code | Units | Levels | ||
|---|---|---|---|---|---|---|
| −1 | 0 | +1 | ||||
| Metal free cascade Reaction | Base used (equivalent) | A | mg | 1 | 2 | 3 |
| Reaction temperature | B | °C | 110 | 120 | 130 | |
| Reaction time | C | h | 4 | 8 | 12 | |
| Entry | Base | Solvent | Temp (°C) | Yield b (%) |
|---|---|---|---|---|
| 1 | - | - | 100 | NR c |
| 2 | - | EtOH | 80 | NR c |
| 3 | Triethylamine | EtOH | 80 | NR c |
| 4 | Na2CO3 | EtOH | 80 | Traces |
| 5 | Piperidine | EtOH | 80 | NR c |
| 6 | NaOtBu | t-BuOH | 100 | 40 |
| 7 | KOtBu | t-BuOH | 100 | 30 |
| 8 | NaOH | EtOH | 80 | 50 |
| 9 | KOH | EtOH | 80 | 56 |
| 10 | KOH | MeOH | 70 | 40 |
| 11 | KOH | DMSO | 120 | 45 |
| 12 | KOH | DMF | 120 | 93 |
| 13 | KOH | 1,4-Dioxane | 80 | 25 |
| 14 | KOH | THF | 80 | 16 |
| 15 | KOH | Acetonitrile | 80 | 16 |
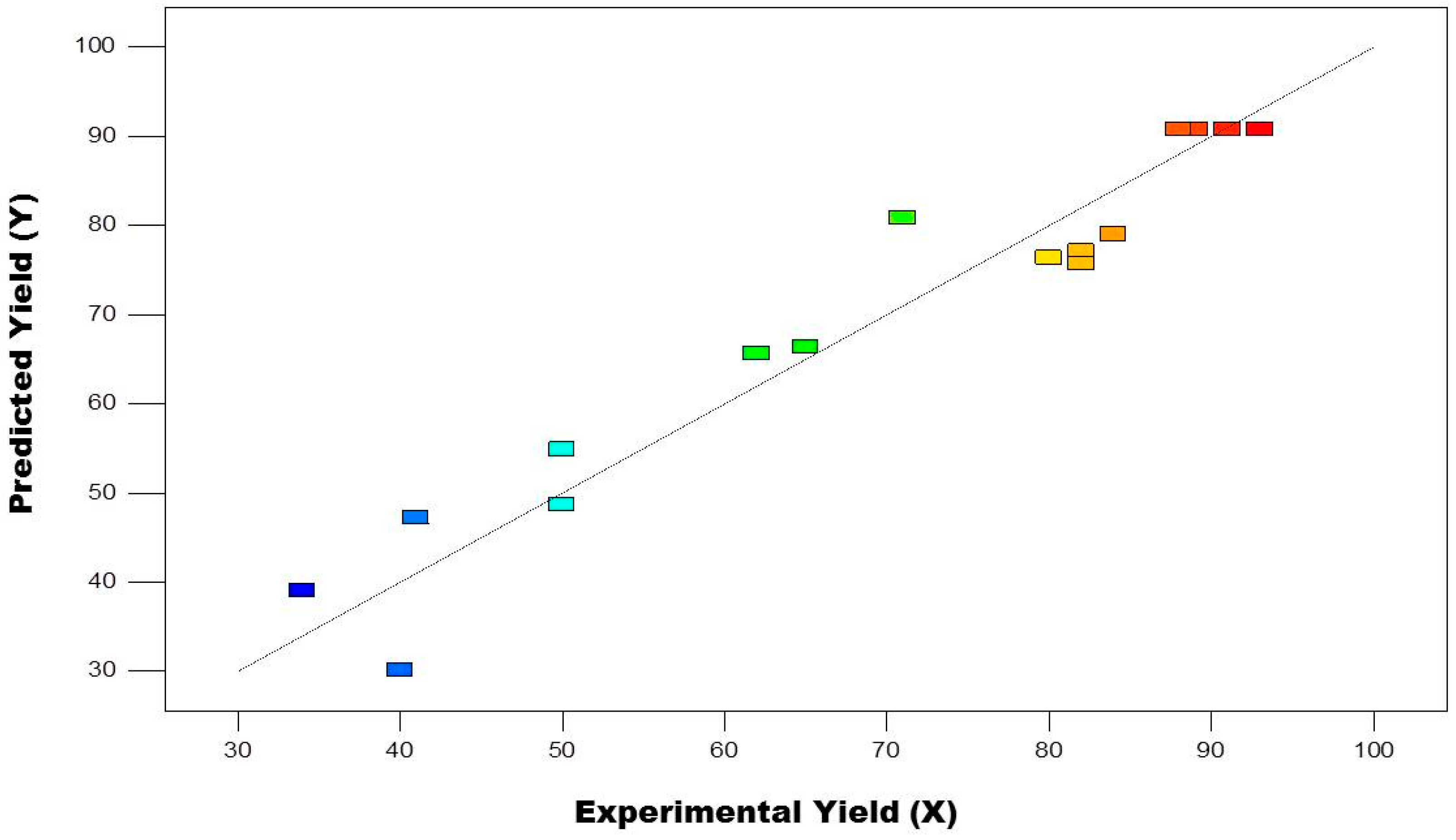
| Run | Metal Free Condition | ||||
|---|---|---|---|---|---|
| A | B | C | Y (%) | X (%) | |
| 2 | 120 | 8 | 93.01 | 90.80 | |
| 2 | 120 | 8 | 89.00 | 90.80 | |
| 1 | 120 | 4 | 40.33 | 30.12 | |
| 1 | 130 | 8 | 62.03 | 65.62 | |
| 3 | 130 | 8 | 82.09 | 77.12 | |
| 2 | 110 | 4 | 34.12 | 39.00 | |
| 3 | 110 | 8 | 80.31 | 76.37 | |
| 3 | 120 | 4 | 50.41 | 48.62 | |
| 2 | 120 | 8 | 91.01 | 90.80 | |
| 3 | 120 | 12 | 71.00 | 80.88 | |
| 1 | 110 | 8 | 50.21 | 54.87 | |
| 2 | 110 | 12 | 82.07 | 75.75 | |
| 2 | 130 | 4 | 41.09 | 47.25 | |
| 1 | 120 | 12 | 65.21 | 66.37 | |
| 2 | 120 | 8 | 88.44 | 90.80 | |
| 2 | 130 | 12 | 84.51 | 79.00 | |
| 2 | 120 | 8 | 93.01 | 90.80 | |
| Source | Sum of Squares | DF | Mean Square | F Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 6271.98 | 9 | 696.89 | 11.57 | 0.0020 | S |
| A | 544.50 | 1 | 544.50 | 9.04 | 0.0197 | |
| B | 66.13 | 1 | 66.13 | 1.10 | 0.3295 | |
| C | 2346.12 | 1 | 2346.12 | 38.96 | 0.0004 | |
| AB | 25.00 | 1 | 25.00 | 0.42 | 0.5399 | |
| AC | 4.00 | 1 | 4.00 | 0.066 | 0.8040 | |
| BC | 6.25 | 1 | 6.25 | 0.10 | 0.7567 | |
| A | 714.32 | 1 | 714.32 | 11.86 | 0.0108 | |
| B | 362.21 | 1 | 362.21 | 6.01 | 0.0439 | |
| C | 1905.79 | 1 | 1905.79 | 31.65 | 0.0008 | |
| Residual | 421.55 | 7 | 60.22 | |||
| Lack of Fit | 400.75 | 3 | 133.58 | 25.69 | 0.0045 | S |
| Pure Error | 20.80 | 4 | 5.20 | |||
| Correlation Total | 6693.53 | 16 |
| Parameter | Base Equivalent (A) | Reaction Temperature °C (B) | Reaction Time (min) (C) | % Yield |
|---|---|---|---|---|
| Predicted | 2.23 | 125.3 | 9.17 | 93.5 |
| Experimental | 2.0 | 120.0 | 8.0 | 93.0 |
 |
| Entry | Λmax (abs, nm) | Λmax (em, nm) | Stokes Shift (nm) | OD | I | ΦF |
|---|---|---|---|---|---|---|
| Tryptophan [32] | 280 | 355 | 75 | 0.384 | 158,517 | 0.130 |
| 4a | 306 | 533 | 227 | 0.774 | 46,182 | 0.019 |
| 4b | 306 | 530 | 224 | 0.957 | 90,759 | 0.031 |
| 4c | 308 | 553 | 245 | 1.064 | 31,279 | 0.010 |
| 270 (Sh) | 553 | 283 | 0.838 | 31,279 | 0.012 | |
| 4d | 310 | 524 | 214 | 1.505 | 115,328 | 0.025 |
| 4e | 300 | 518 | 218 | 0.870 | 165,016 | 0.062 |
| 344 (Sh) | 518 | 174 | 0.788 | 165,016 | 0.068 | |
| 4h | 310 | 524 | 214 | 1.505 | 115,328 | 0.025 |
| 4i | 306 | 534 | 228 | 1.883 | 73,195 | 0.013 |
| 270 | 534 | 264 | 1.251 | 73,195 | 0.019 | |
| 4j | 300 | 534 | 234 | 1.140 | 61,312 | 0.017 |
| 4k | 314 | 532 | 218 | 1.950 | 83,407 | 0.014 |
| 4l | 320 | 540 | 220 | 2.070 | 65,088 | 0.010 |
| 4m | 308 | 535 | 227 | 1.794 | 70,535 | 0.013 |
| 4n | 272 | 530 | 258 | 0.449 | 54,760 | 0.040 |
| 322 (Sh) | 530 | 208 | 0.439 | 54,760 | 0.041 | |
| 4o | 270 | 543 | 273 | 1.761 | 29,458 | 0.005 |
| 4p | 306 | 499 | 193 | 1.176 | 59,599 | 0.016 |
| 268 (Sh) | 499 | 231 | 0.827 | 59,599 | 0.023 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palaniraja, J.; Mohana Roopan, S.; Mokesh Rayalu, G.; Abdullah Al-Dhabi, N.; Valan Arasu, M. A Metal-Free Regioselective Multicomponent Approach for the Synthesis of Free Radical Scavenging Pyrimido-Fused Indazoles and Their Fluorescence Studies. Molecules 2016, 21, 1571. https://doi.org/10.3390/molecules21111571
Palaniraja J, Mohana Roopan S, Mokesh Rayalu G, Abdullah Al-Dhabi N, Valan Arasu M. A Metal-Free Regioselective Multicomponent Approach for the Synthesis of Free Radical Scavenging Pyrimido-Fused Indazoles and Their Fluorescence Studies. Molecules. 2016; 21(11):1571. https://doi.org/10.3390/molecules21111571
Chicago/Turabian StylePalaniraja, Jeyakannu, Selvaraj Mohana Roopan, G Mokesh Rayalu, Naif Abdullah Al-Dhabi, and Mariadhas Valan Arasu. 2016. "A Metal-Free Regioselective Multicomponent Approach for the Synthesis of Free Radical Scavenging Pyrimido-Fused Indazoles and Their Fluorescence Studies" Molecules 21, no. 11: 1571. https://doi.org/10.3390/molecules21111571
APA StylePalaniraja, J., Mohana Roopan, S., Mokesh Rayalu, G., Abdullah Al-Dhabi, N., & Valan Arasu, M. (2016). A Metal-Free Regioselective Multicomponent Approach for the Synthesis of Free Radical Scavenging Pyrimido-Fused Indazoles and Their Fluorescence Studies. Molecules, 21(11), 1571. https://doi.org/10.3390/molecules21111571